
Chemical stories rarely unfold on a straight path. Calcium isooctanoate took its place in the modern chemical industry during the late 20th century, timed along with growing demand for specialized salts to serve plastics, lubricants, and surface treatments. Before this, the focus stuck closely to more basic calcium soaps, often derived from easily accessible fatty acids. Engineers and chemists searching for ways to shape reaction flow, improve stability, and give coatings longer life began tinkering with “branched” carboxylate structures. Isooctanoic acid, with its eight carbon backbone and irregular structure, filled the need for a less volatile, more temperature-stable acid source. Once paired with calcium, it unlocked possibilities that straight-chain calcium salts couldn’t reach, especially for PVC stabilization and anti-caking blends. This material didn’t show up by accident. Its emergence says a lot about how necessity pushes small innovations that change bigger pictures—one salt at a time.
On store shelves and in chemical drums, calcium isooctanoate rarely gets mentioned by name. Most folks in plastics plants or asphalt labs might know it as a white powder or waxy solid, feeding a mixer alongside other exotic-sounding compounds. The product stands out for being neither truly oily nor chalk-like. It gets used in PVC cable compounds, plasticizers, paint driers, and some specialty greases that don’t like seeing usual metallic soaps. It handles heat better than a straight calcium stearate, thanks to its branched isooctyl tail. Suppliers push it as a dust-free, low-bulk-density additive, with granules or chips that don’t cake up like others. Still, its real value comes into play when users need the calcium cation without messing up the plastic’s finish, or when basic lubricity falls short in complicated blends.
Hold a sample up to the light and you see a soft, easy-to-crumble white material with barely any smell. Its melting point usually lands somewhere between 140-160°C, putting it in a comfortable zone for plastic compounding but far from mineral waxes in toughness. Solubility stays low in water and high in non-polar solvents or oils, which means it’s unlikely to dissolve away in damp conditions. Unlike its cousins like calcium stearate, this salt’s branched chains give it better dispersibility in certain polymers and better resistance to surface oxidation. The substance’s density ranges near 1 gram per cubic centimeter, not much different from other calcium soaps, but its low volatility and mild alkaline reaction under water set it apart in specialty fields.
Distributors supply calcium isooctanoate under clear-cut specs. Purity usually exceeds 98% (typically measured by gravimetric calcium content and acid value methods). Moisture content stays under 2%, guarding against clumpy drums and inconsistent batching. Particle size helps processors predict how smoothly it’ll blend: some brands supply fine powders under 100 microns, while others stick to larger granules above 500 microns to keep dust levels down. Good labeling includes CAS number (often 15827-60-8), batch codes, recommended shelf life of two years, and hazard statements that point to the compound’s general low-risk status. Every sack or drum should include lot tracking data, crucial for audits in industries such as food packaging or electrical insulation.
Most plants start with isooctanoic acid, itself made from oxidation of hydrocarbons like isooctane or from fermentation routes using engineered microbes. Producers combine purified isooctanoic acid with calcium hydroxide or calcium oxide, usually in a heated reactor with careful pH monitoring. The process runs best with a little excess base, ensuring all the acid reacts to give off water and solid salt. Wet cake gets filtered, washed to remove traces of unreacted acid, then dried in vacuum ovens or spray dryers. Depending on the intended market, the final form can be milled to a powder or pressed into flakes for better handling.
Chemically, the base reaction forms a neutral salt with two isooctanoate groups for every calcium ion. Under heat, the substance resists breaking down, unlike unmodified calcium soaps that start to yellow or produce odors at similar temperatures. Mixing with other metallic salts, such as zinc or magnesium carboxylates, can create new synergistic additives for lubricants and cable compounds. Some labs also explore partial substitution of isooctanoic acid with other branched acids to further tweak melting points and dispersibility. Acid exchange reactions open doors to making calcium isooctanoate from existing calcium stearate stocks, offering a route to recycle waste from other processes.
Beyond its IUPAC name, folks use terms like “calcium 2-ethylhexanoate,” “calcium caprylate,” and “calcium isooctylate.” Some catalogues list it as “Calcium bis(2-ethylhexanoate),” especially in high-purity versions. In the paint driers market, it turns up as “calcium octoate.” Producers push trademarked blends that blend calcium isooctanoate with other fatty acid salts under proprietary names—each chasing better stability or process smoothness for target industries. On safety data sheets, expect to see synonyms and CAS numbers lined up to guide customers past mixing it up with other calcium soaps.
Nobody likes surprises in the workplace. This compound, stemming from food-compatible fatty acids and inert calcium, carries a low hazard score. Skin and eye irritation reports stay rare except in unusually dusty settings, so standard gloves and goggles suffice for almost every application. Overexposure through inhalation can kick up routine mild symptoms for folks with allergies or chronic respiratory conditions, but nothing out of line with most factory powders. International standards—like REACH in Europe—demand tracking by batch, routine impurity testing, and detailed exposure guidelines on labels. Disposal follows standard non-hazardous waste protocols, although some local rules prefer solidification or composting when used in agricultural blends.
Long afternoons on the plant floor show just how valuable this salt turns out for plastic manufacturers. PVC cable insulation, flexed daily in electronics, resists heat aging much better with this additive than with generic calcium stearate alone. Asphalt labs add it to improve binder flow in paving compounds. Paint chemists rely on it in drier blends to keep films tough against the elements. Lubricant makers blend it into specialty greases, chasing higher drop points and stickier films, especially where contact with water turns standard calcium soaps into a mess. The food packaging sector uses it only in indirect-contact roles, counting on calcium isooctanoate’s low migration and high temperature resistance to meet regulatory pressure.
Research groups dive into how small tweaks in the acid’s branching change melt profiles, resistance to oxidation, and compatibility with plant-based polymers. A growing theme explores using bio-based isooctanoic acids, partly to shrink the carbon footprint and cut ties with petroleum supply swings. Other studies focus on making particle sizes more uniform for 3D printing blends and electronic encapsulants. Collaborative work with universities opens new paths: stabilizing recyclable PVC, developing lubricants for wind turbines and electric vehicles, and testing new food-safe coatings where metal migration matters. Some teams chase answers for faster, lower-energy production methods, targeting greener routes to match shifting regulations.
Toxicological studies run deep for both components—calcium ions and isooctanoates. Repeated testing supports low oral and dermal toxicity. Acute exposures in animals rarely produce significant symptoms at realistic levels, and no established links exist to long-term organ damage or cancer. Overexposure through inhalation could cause coughing and mild respiratory issues, but workplace controls reduce this risk. Regulatory eyes zero in on any chance of migration into foods, especially for packaging applications. In long-term studies, neither breakdown products nor the compound itself have shown bioaccumulation or persistent environmental toxicity. Environmental monitoring in heavily industrialized areas hasn’t flagged calcium isooctanoate as a concern for waterways or soil.
Looking ahead, calcium isooctanoate stands to grow in areas where regulators watch migration and plastic recyclers demand additives with lower environmental impact. Greener synthesis looks set to cut process emissions, likely powering research into fermentation-based isooctanoic acid. Designers tinkering with flexible solar panels, wearable electronics, and food-safe films start with this molecule as a stabilizer of choice. Cross-disciplinary feedback, from automotive labs to high-voltage cable plants, promises a broader toolbox for compounders. New market needs—slower aging, less migration, improved processability—push chemists to keep tweaking molecular shapes, pushing calcium isooctanoate blends far beyond their humble early beginnings in the basic chemistry set.
Calcium isooctanoate doesn’t turn heads in the grocery aisle, yet this compound pulls a lot of weight behind the scenes. Most people probably don’t realize that it shows up in many foods—not for taste, but for staying power and structure. Food manufacturers use calcium isooctanoate to keep things mixed together and stable, especially in baked goods and processed products. Without stabilizers like this, sandwich bread or cake mixes would fall apart on the shelf.
In my own kitchen, I’ve watched homemade cakes get dense or gooey if I skip certain steps. Big factories don’t have the luxury of baking from scratch every day, so calcium isooctanoate fills that gap. Its main job is to hold ingredients together and prevent them from separating in transit or storage. Consumers mostly care about texture, freshness, and that familiar sliced bread feel, which is exactly where this additive shines.
Beyond bread, calcium isooctanoate shows up in cheese, non-dairy creamers, and even snacks advertised as “fortified.” Cheese production uses it to help manage the presence of molds or to achieve a steady texture batch after batch. In vegan cheese and milk-alternatives, the need becomes even clearer: plants and oils don’t blend smoothly by themselves, so this additive provides a structure that people expect from animal-based options.
The global snacking trend means more processed foods, often traveling long distances. Nobody wants to bite into a chalky cracker or sip a gritty smoothie. The food industry depends on flow agents like calcium isooctanoate to keep things palatable and safe, especially if the food could clump up or separate under pressure or heat. Many nutrition bars wouldn’t maintain shape in a backpack or warm car without some kind of stabilizing agent.
Supplements often look simple—just a pill or capsule. What goes inside takes technical know-how. Calcium isooctanoate can assist as an excipient, acting sort of like the mortar in a brick wall, holding active ingredients together. This matters for both reliable dosing and stability over time.
Personal experience selling vitamins in a health food store taught me that people expect their supplements to stay intact, right up to the expiration date. The supplement business uses additives to deliver the nutrients promised on the label, and to do that, capsules have to avoid sticking to each other or crumbling. Since calcium is necessary for people who are lactose-intolerant or vegan, safe sources of calcium—sometimes delivered through isooctanoate compounds—become even more crucial.
Concerns about additives are fair. Nobody wants harmful chemicals hidden in their food. Regulatory bodies like the FDA review these compounds, testing safety and setting limits. As of now, no evidence links calcium isooctanoate in approved concentrations to health risks. Still, clearer labels and transparency keep people informed. Personally, I don’t want to memorize an ingredients dictionary just to shop for groceries.
The bigger conversation circles back to trust. Trust demands high standards in food safety, honest labeling, and options for those looking to avoid additives. Support for transparent manufacturing and better scientific research helps shoppers make confident choices, whether they’re scanning snack packs or talking to their pharmacist.
Walking down grocery aisles, ingredient lists tend to throw out names that sound more like chemistry finals than actual food. Among them, Calcium Isooctanoate has started showing up in technical documents related to food production. The name doesn't exactly roll off the tongue, and folks naturally want to know just what it might do in their snacks or supplements. As someone who pays attention to what goes into daily meals, the question of safety stands out. So, how do we break down the truth about Calcium Isooctanoate?
Calcium Isooctanoate comes from combining calcium with isooctanoic acid, a branching fatty acid otherwise seen in various animal fats and some plants. Food manufacturers look at it for technical uses, such as an emulsifying agent or stabilizer. That means it could help textures or keep ingredients from separating, letting foods stay fresher and look more appetizing on store shelves.
Many calcium compounds act as food additives already — from calcium carbonate in antacids to calcium chloride in tofu. The key, though, isn't to trust something just because it's "calcium." Each new compound deserves its own close look. That's especially important when dealing with anything that could become a common additive.
Regulatory agencies such as the FDA in the United States, EFSA in Europe, and food safety groups around the world look closely at new additives. They check for toxicity, allergic reactions, and how our bodies process the compound. For Calcium Isooctanoate, published safety data remain thin, with few large independent studies and no conclusive green light from major agencies.
One European Food Safety Authority (EFSA) assessment found current evidence on this additive limited; without significant animal or human study, they held back from making a confident call. Usually, substances only end up on approved lists after they pass through long-term exposure tests. These spot signs of chronic toxicity, genetic risks, or odd interactions with other foods and medicines. At this point, Calcium Isooctanoate lacks the volume of research received by older, more familiar additives.
My own kids are picky eaters, but I've learned to keep a sharper eye on what ends up in their food. Just because a name looks impressive—or sounds like something you'd find in multivitamins—doesn’t mean it belongs in every meal. Food companies have a knack for using technical-sounding ingredients to solve manufacturing challenges, but their priorities don’t always line up with long-term public health.
Looking at the data so far, it's hard to comfortably recommend Calcium Isooctanoate for regular diets. Health has no rewind button, and the body responds in ways even experts can't always predict when they're dealing with new compounds. Without enough testing, people who are sensitive to additives—such as those with allergies or digestive issues—sit at increased risk. For those with calcium-deficiency concerns, safer, well-researched sources like dairy products, leafy greens, or established supplements keep meals both nutritious and reliable.
Safer food depends on demanding transparency and enough testing from regulators and manufacturers alike. It's time to ask hard questions before anyone introduces a new chemical into supermarkets. Only consistent, peer-reviewed research lets families make informed choices, supported by trust and transparency rather than wishful thinking or marketing jargon. Until that day, cutting back on processed foods and sticking with ingredients that have stood the test of time gives all of us the best shot at safety.
Every so often, an ingredient grabs my attention and makes me wonder why it doesn’t show up on more lips. Calcium isooctanoate has done just that, and for reasons that go beyond simple chemistry. Whether formulating plastics, paints, or lubricants, anyone who’s spent time on a production line knows that every additive earns its place. Calcium isooctanoate does this quietly but with impressive results.
Sometimes, getting ingredients to blend smoothly and consistently feels like trying to make oil and water shake hands. One season on a plastics line, I watched operators battle stubborn clumps and uneven dispersions that cost hours of lost productivity. Introducing a metal soap like calcium isooctanoate usually leads to a smoother melt flow. The polymer won’t burn or discolor as quickly, and the end result often comes out with fewer streaks or spots. That’s not just a win for the bottom line, but for the people doing the tough work in hot, noisy environments where every snag stings.
Years working around industrial paints taught me to appreciate what a good drier does for an alkyd system. Calcium isooctanoate steps up as a reliable auxiliary drier. It helps accelerate curing, keeps film formation on track, and reduces the risks of tacky surfaces that attract dust or fingerprints. These aren’t hypothetical lab scenarios; I’ve seen clients relieved to ship goods on schedule, no longer wrestling with sticky finishes. It also means fewer calls complaining about paint that feels soft days after application. Real-world results like these keep shops humming and reputations intact.
In metalworking or mold-release applications, everything comes down to friction—or more importantly, how to reduce it. On crowded factory lines, equipment downtime hits hard. Operators face fewer shutdowns since calcium isooctanoate acts as a dependable lubricant, letting machines glide instead of grind. Components last longer and require less maintenance. It’s not just about smoother performance, but increased safety, since heat and friction add up to real hazards. This kind of additive won’t make headlines, but people who work with anything from die-cast parts to moving conveyer belts can vouch for what it adds to their day.
Through the years, regulatory standards keep tightening, especially around emissions and worker exposure. Calcium isooctanoate, being less toxic than many heavy-metal alternatives, helps companies meet compliance goals without sacrificing product quality. I’ve talked with safety managers grateful for options that keep teams healthier and reduce the burden of hazmat paperwork. Every chance to pick a safer ingredient puts people ahead—and that matters.
Back in the supply office, I’ve seen decision-makers compare the long-term value of materials. More stable performance, longer equipment life, and fewer process interruptions all add up. Calcium isooctanoate often gives solid value without the price swings seen with raw metals or rare additives. Reliable sourcing helps maintain predictability in a world that rarely feels predictable, from my past experience talking to procurement teams always hunting for dependable supply partners.
Smart choices in raw materials ripple out across the whole production chain. Calcium isooctanoate delivers practical improvements in handling, quality, and safety. That keeps both products and the people who make them moving forward.
Calcium isooctanoate turns up in food science conversations as a calcium salt of isooctanoic acid, used in some food applications and considered for potential health supplements. Curiosity about any substance we eat or use isn’t just smart—it’s essential for our well-being. Lots of folks ask whether it brings any side effects. This isn’t just a matter of curiosity; it’s about knowing what we put into our bodies and keeping our health on track.
Looking to trusted reports and scientific papers, calcium isooctanoate hasn’t gotten the same level of scrutiny as more common calcium salts, like calcium carbonate or citrate. Public databases and research journals don’t offer up many results about this compound’s side effects. This should sound a little alarm: lack of robust human trials means fewer answers about how bodies respond, especially for those with allergies or underlying health issues.
No major regulatory authority—like the FDA or European Food Safety Authority—has published reports highlighting concrete hazards tied directly to calcium isooctanoate in food or supplement form. Food safety reviews often lump this compound together with other calcium salts, leaning on the idea that it breaks down into ions and fatty acids the gut already meets. But the reality is that its unique structure might behave differently, especially with long-term use.
One person’s harmless supplement can be another person’s allergy trigger. Calcium on its own sometimes brings constipation, bloating, and even kidney stones for those who overdo it. The attached isooctanoate tail isn’t a chemical commonly found in the typical diet, which means digestion could sometimes go sideways—mild stomach upset, diarrhea, or discomfort might show up if the gut isn’t ready for it.
Personal experience speaks volumes. People sensitive to fatty acids sometimes notice skin rashes or mild digestive symptoms when they try new food additives. With calcium isooctanoate, we don’t find a long trail of reported problems, but that probably says more about the lack of widespread use than about proven safety.
True safety isn’t about the absence of warnings; it’s about real transparency, long-term data, and trust. This compound doesn’t share the decades-long track record that others do. Before mixing it into foods or taking it as a supplement, it’s smart to check with a medical professional, especially for those with kidney trouble, allergies, or special dietary needs.
Risk can come from how much someone consumes, underlying health status, and even how other parts of the diet interact. For now, most users would be better off with forms of calcium well-researched and widely accepted by doctors. If a company or researcher wants to introduce new additives like this one, clear labeling and more public studies matter. Without visible data, we can’t just accept a “generally safe” label at face value.
Safer nutrition comes down to doing homework before swapping in new ingredients. Companies working with calcium isooctanoate should share detailed data, especially long-term studies in real people. For the everyday shopper or supplement user, sticking with known, trusted sources of calcium makes sense until there’s more information on this chemical’s track record.
Health comes from informed choices, not guesswork. The next time a new supplement or additive pops up, it’s important to pause, do a little extra research, and have an honest talk with someone who knows both the science and personal medical history. Sometimes, caution is the best self-care.
Calcium isooctanoate doesn’t jump out in news stories like famous elements or common household chemicals, but people working in chemical labs and manufacturing deal with it enough to know shortcuts can cause real trouble. This compound appears in plastic stabilizers and lubricants. It’s not flammable or shock sensitive, so it doesn’t demand a bunker or safety drama, but sloppy storage ruins product, invites contamination, and puts teams at risk.
I’ve seen far too many chemical cabinets where packaging looks good on day one, but humidity or warmth ruins the contents by month three. Calcium isooctanoate likes a cool, dry spot. I’ve seen labs hit problems in summer when their storeroom heats up and the chemical clumps or cakes. Chemical suppliers often stress a range from 15°C to 25°C. Ignore it, and material can change in texture or get stuck, dragging down process efficiency. Nobody should lose an afternoon scraping out a drum or shaking a bottle because the storeroom acted like a sauna for two weeks.
Any chemist worth their salt checks for moisture before opening the storage area in the morning. There’s nothing mysterious about this: water in the air drifts through torn bags or cracked lids, which can start hydrolyzing the calcium salt, creating a mess. Silica gel packets and tight screw caps help, but don’t always rescue badly handled stock. A lot of warehouses use a simple digital hygrometer for real oversight. These cheap tools let anyone spot issues before chemicals go bad.
Sometimes colleagues act casual about chemical choice for containers. I’ve seen people pour calcium isooctanoate into open-topped bins or re-use soda bottles. That kind of shortcut risks spills and introduces junk from other chemicals. Best practice uses sealed plastic drums or HDPE containers, labeled right and kept away from reactive metals. Don’t underestimate the problems that come from contamination. It only takes a little grit or dust from a neighboring chemical to change how this additive behaves in a mix, and I’ve witnessed entire process batches sent for disposal after something unexpected showed up because of sloppy container choice.
Calcium isooctanoate shouldn’t sit near acids or oxidizers. I remember a situation in a shared space where an unmarked bottle ended up next to a box of bleach, and vapors mixed in a way that set off detectors. It wasn’t catastrophic, but it was an expensive lesson in common sense storage and labeling.
Date your containers. I get why people skip this—busy day, quick job, but it leads to guesswork six months later. Suppliers often recommend using oldest stock first. Digital inventory tracking keeps teams honest and clears up old, compromised batches before they spill into production. No chemical lasts forever—good inventory means less waste.
People sometimes treat less-famous chemicals as an afterthought, but experience shows the ugly side of treating storage as a back-burner issue. Calcium isooctanoate deserves respect. Safe, organized storage saves money and guardrails health, especially for folks working in warm, humid climates or at smaller operations without climate controls. Small habits build the kind of reliability that keeps teams safe and products consistent.
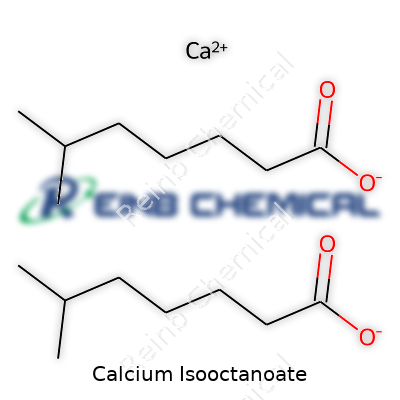
| Names | |
| Preferred IUPAC name | Calcium 4-methyloctanoate |
| Other names |
Calcium 2-ethylhexanoate Calcium octoate Calcium 2-ethylhexylate |
| Pronunciation | /ˈkæl.si.əm aɪ.soʊˈɑk.təˌnoʊ.eɪt/ |
| Identifiers | |
| CAS Number | [CAS: 40457-84-1] |
| Beilstein Reference | 1901305 |
| ChEBI | CHEBI:85147 |
| ChEMBL | CHEMBL4354762 |
| ChemSpider | 32975352 |
| DrugBank | DB11121 |
| ECHA InfoCard | ECHA InfoCard: 100.240.902 |
| EC Number | 275-158-7 |
| Gmelin Reference | 607737 |
| KEGG | C18799 |
| MeSH | D017687 |
| PubChem CID | 10313576 |
| RTECS number | RR0350000 |
| UNII | HBW8L77ZX8 |
| UN number | UN3082 |
| CompTox Dashboard (EPA) | display/CASRN/142-31-4 |
| Properties | |
| Chemical formula | Ca(C8H15O2)2 |
| Molar mass | 342.52 g/mol |
| Appearance | White powder |
| Odor | Odorless |
| Density | 0.98 g/cm³ |
| Solubility in water | Insoluble |
| log P | 1.89 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 10.0 |
| Basicity (pKb) | 12.2 |
| Refractive index (nD) | 1.451 |
| Viscosity | 550 mPa·s (20°C) |
| Dipole moment | 2.9627 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 862.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1058.7 kJ/mol |
| Pharmacology | |
| ATC code | A12AA20 |
| Hazards | |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H318: Causes serious eye damage. |
| Precautionary statements | IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 150°C |
| Lethal dose or concentration | LD50 Oral Rat: >2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral >5000 mg/kg |
| NIOSH | Not Established |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.5-2% |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Isovaleric acid Isooctanoic acid Calcium valerate Calcium octanoate |