
Cerium Isooctanoate, a little-known but essential organocerium compound, didn’t simply arrive overnight. Chemists first began exploring rare earth organometallic salts in the early-to-mid 20th century, largely because these compounds offered new routes in catalysis and materials science. Cerium, a member of the lanthanide series, stood out due to its chemical reactivity and abundance compared to its peers. The path to cerium isooctanoate began with early efforts to make cerium carboxylates practical, driven largely by industrial demand for more efficient paint dryers and fuel additives during the decades after World War II. Manufacturers who developed cerium soaps saw them as cleaner alternatives to lead-based driers. They tinkered with different fatty acids and hydrocarbon chains before settling on isooctanoate as a workable option — balancing cost, performance, and storage stability. Experience in chemical plants over the years showed that the formulation of cerium isooctanoate could withstand batch process variation, with better solubility in organic media than many of its cousins. That history explains how cerium isooctanoate evolved into a staple for a few specialist applications.
Cerium isooctanoate isn’t sold off the shelf in ordinary hardware stores, but it’s crucial for manufacturers who need a strong metal-based catalyst. This compound exists as a cerium salt of 2-ethylhexanoic acid, typically dissolved in aromatic hydrocarbons. Chemically, it delivers cerium in a form that disperses well in organic solvents, a key feature for both paint recipes and fuel additives. Companies produce it as a viscous pale yellow liquid with a faint oily smell. Packaging standards call for sealed barrels or drums since the liquid reacts with moisture and air over time. Quality control relies on tightly maintained metal concentrations and acid values, since even small shifts affect performance. From my time working with catalysis researchers, I have learned that reproducible results depend on sourcing consistent product batches.
The physical profile looks straightforward on the surface but hides a few quirks. Cerium isooctanoate’s melting point sits well below room temperature, so it refuses to crystallize under most storage conditions. The liquid is usually slightly yellow, hinting at its cerium content. With a density above most hydrocarbons, it settles quickly in mixtures if left undisturbed. In terms of solubility, cerium isooctanoate dissolves in xylene, toluene, High Flash Naphtha, and similar organic solvents; it rejects water. Chemically, cerium centers remain in the +3 oxidation state, coordinated by two or more isooctanoate ligands, and the overall compound resists decomposition under mild heating. Handling the bulk material calls for gloves, goggles, and a well-ventilated area. Mixing or diluting the compound in a lab requires careful attention because vapors from the solvent base can irritate airways.
Suppliers who provide technical information on cerium isooctanoate typically report the cerium metal content as a percentage by weight, alongside the acid number, water content, and viscosity at a defined temperature. It’s critical that workers read and understand the safety data sheet (SDS) before use; some suppliers use a variety of synonymous product names or even trade designations, so relying on CAS numbers helps confirm identity. Transport labels usually warn of flammable and irritant risks, with clear directions for storage away from direct sunlight, oxidizers, and acid-sensitive chemicals. Any batch purchased for industrial use should come with a certificate of analysis tracing its elemental composition and solvent content. I have seen manufacturers refuse delivery on shipments that fall out of spec — it’s an expensive lesson to learn if you don’t pay attention to the labeling details upfront.
Producing cerium isooctanoate starts with cerium oxide or cerium carbonate as the base material. Chemical engineers dissolve this cerium salt in a dilute acidic solution of 2-ethylhexanoic acid under controlled temperatures. After reacting the raw materials for several hours, leftover water and unreacted acid are stripped from the mix, usually under vacuum, to give a thick solution of cerium isooctanoate in an organic solvent. Plant operators keep a close eye on pH, moisture, and temperature throughout the run — miscalculations can cause side-reactions that lower metal purity or introduce water, ruining storage stability. I once helped troubleshoot a batch where the solvent washed out excessive unreacted acid, making the formulation too acidic for final use in coatings; small changes in process design prevented future headaches.
One standout feature of cerium isooctanoate is how it behaves with peroxides and unsaturated hydrocarbons. In paint and varnish applications, it acts as a catalytic drier by accelerating the oxidative cross-linking of drying oils. That’s possible because cerium shifts between different oxidation states, trading electrons in a way that normal carboxylates cannot. Labs have tested modifications that swap out part of the isooctanoate ligands for other fatty acid chains, adjusting solubility and reactivity for specialty coatings or lubricants. Adding partners like cobalt or zirconium carboxylates can tune performance even further, letting chemists fine-tune hardness, drying speed, or gloss. In fuel chemistry, cerium isooctanoate assists in breaking down soot and particulates by stabilizing radical intermediates; research continues on ways to blend it with other metal additives to amplify these effects.
Cerium isooctanoate pops up under a handful of synonyms, which makes tracking chemical information tricky for newcomers. Industry references sometimes use cerium 2-ethylhexanoate, cerium caprylate, or just “cerium octoate” on their paperwork. Branded versions may carry trade names like Additek Ce-8 or EHCerate, depending on the vendor. Chemists must pay special attention because these labels can hide small but important differences in formulation — some “octoates” use linear, not branched, carboxylic acids. I recall a technical project where switching between two “cerium octoates” from separate suppliers led to uneven drying rates in alkyds, sparking a preventable round of troubleshooting.
Everybody handling cerium isooctanoate relies on standardized safety practices for organometallics. This isn’t a toxic bomb, but the solvent base and metal salt can still trigger headaches, skin irritation, or worse if handled carelessly. Workspaces keep up-to-date SDSs close at hand. Good ventilation, tightly sealed containers, and solvent-rated gloves form the backbone of safe handling. Storage rules focus on keeping drums cool, dry, and away from sources of ignition. Spill response hinges on absorbent pads and immediate removal to hazardous-waste collection sites, since neither the cerium salt nor the residual isooctanoic acid is kind to groundwater or soil. Long exposure to fumes or splashes can cause harm, so companies train their teams to catch small leaks and cleanups early. From what I have seen, most accidents happen when workers grow complacent — ongoing training and refreshed signage help reduce these common errors.
Paint and surface coatings companies value cerium isooctanoate for its role as a drier in alkyd-based systems. Unlike cobalt, which raises increasing health concerns, cerium has a better environmental profile and works as a secondary drier by boosting peroxide decomposition in paint films. Adding cerium isooctanoate to a varnish recipe cuts down on drying time and improves color retention, especially in thick or alkyd-rich layers. The fuel industry injects it into diesel blends to slash soot formation and enhance combustion efficiency by providing an oxygen reservoir right at the flame front. Research groups studying emissions from heavy trucks report lower particulate residue when cerium-based additives supplement traditional fuel blends. A handful of niche applications include lubricants, printing inks, and even synthetic resin curing, where cerium’s high redox swing supports rapid polymerization. These uses may not appear in every plastic or paint factory, but companies that have tried the additive usually stick with it for the performance gain.
Academic and industrial labs turn to cerium isooctanoate for research into alternative drier systems, low-VOC coatings, and next-generation fuel additives. Chemists test new ligand blends and metal ratios seeking cleaner, more controllable drying in paints. Some work looks at swapping out the hydrocarbon solvents entirely for greener, low-toxicity carriers, a vital step as environmental standards tighten. A strand of research digs into the catalytic pathways that let cerium accelerate cross-linking in fatty acid polymers, hoping to create next-gen driers with adjustable curing profiles. Engine developers and emissions specialists are evaluating how cerium additives influence real-world soot suppression under varied engine loads and temperatures. The line between fundamental research and commercial product development is thin here; progress in the lab quickly finds its way into manufacturing or field trials. Industry-academic collaboration speeds up breakthroughs, but the learning curve can slow practical rollout.
The long-term safety record of cerium isooctanoate looks positive compared to cobalt and lead driers. Medical researchers have tracked its acute and chronic effects in animal studies and exposure models. The main health risks, based on evidence from skin and inhalation exposure studies, include eye and skin irritation, and reversible respiratory discomfort at high vapor concentrations. Studies show little tendency for cerium from such sources to bioaccumulate, although the organic solvents in commercial preparations remain a bigger health concern in day-to-day use. Results from multi-year occupational health studies in pigment and paint-additive plants suggest minimal systemic toxicity when modern ventilation and protective equipment are in place. Yet, regulatory agencies keep a close eye on rare earths in general, since industrial dust and off-spec waste could raise environmental or health problems if processing standards slip. Continuing toxicity research stays high on the agenda for both public agencies and chemical suppliers — new solvent systems and formulations may raise fresh questions as trends in green chemistry push for safer alternatives.
Demand for cerium isooctanoate won’t disappear anytime soon, but the chemical sector is ready for change as regulations and technology advances. Increased scrutiny of cobalt-based driers pushes coatings companies to invest in better-performing lanthanide alternatives, and cerium sits at the top of that list. Those leading R&D in green chemistry are seeking ways to lower VOCs and reduce environmental risk by tweaking the solvent blends used in metal carboxylate products like cerium isooctanoate. Growth in the fuel-additive space keeps driving research into improved soot suppression catalysts, firmly keeping cerium products relevant for years to come. Public and regulatory attention on all rare earth supply chains could shift sourcing and processing methods in the future, as transparency and sustainability gain importance. I have seen firsthand how quickly industrial standards move once a proven environmental or economic benefit shows up — if cerium compounds keep outperforming legacy alternatives while meeting safety requirements, their prospects only brighten.
Cerium isooctanoate doesn’t make headlines, but it quietly supports progress in industries ranging from auto manufacturing to energy applications. Most people walk past shiny cars and never think twice about the chemistry behind gloss and protection, but cerium compounds hide in plain sight, playing a practical role in how products last and look on store shelves and in driveways.
Over the years, I’ve worked with folks designing corrosion-resistant coatings. They often face the challenge of keeping metals safe from rust and wear – not just for looks, but for safety and value. Cerium isooctanoate is one of those metal carboxylates that’s gone a little underappreciated in paint additives, but it earns its place by helping paints protect surfaces. Mixed into alkyd or other oil-based paint formulas, this compound acts as a drier, boosting the rate at which solvents evaporate and resins solidify. On a chilly morning or a humid day, when typical formulas might flounder, a touch of cerium can bring reliability and durability.
Tests have shown that paints fitted out with cerium-based driers resist yellowing more than standard cobalt driers. They keep their finish longer, and as regulatory standards tighten on heavy metals like cobalt and lead, cerium fills the gap as a safer alternative. That matters to families repainting their homes and maintenance teams tasked with protecting factories and public infrastructure.
On the industrial side, cerium isooctanoate finds its place in catalyst systems. Anyone who’s followed air quality rules knows auto exhaust drives innovation, and cerium’s chemical talent comes into play here. Used in catalytic converters, cerium isooctanoate helps control emissions, especially nitrogen oxides and carbon monoxide, which sneak past unassisted systems.
Cerium's knack for storing and releasing oxygen boosts the converter’s efficiency during the constant push and pull of urban driving. This doesn’t just keep air cleaner for city dwellers; it stretches the life of catalytic converters, saving money and hassle for car owners. As electric cars nudge into the spotlight, the lessons learned here still ripple outward, teaching researchers how rare earth compounds improve surface chemistry and reactivity beyond gas engines.
Environmental and health regulations keep tightening, rightly so, and that’s where cerium isooctanoate steps forward. Unlike cobalt and lead, which bring a dark history of industrial pollution and health risks, cerium’s toxicity profile looks far more manageable. Chemical safety data lines up with what’s seen in the field: less contamination, less risk for paint workers and customers alike.
Lab studies and practical results both show that switching to cerium-based driers and catalysts can cut down hazardous waste and open doors for recyclable, lower-impact products. The switch isn’t just about rules – it’s also about future-proofing industries that want to keep running smoothly as consumer demands and laws keep shifting.
From my experience working with technical teams, manufacturers look to cerium isooctanoate when older compounds fall short on safety or performance. Costs, handling, and supply chains still bring challenges. Some companies experiment with blends or push for higher-purity sources to meet international demands, but every step brings them closer to a smarter, safer product set.
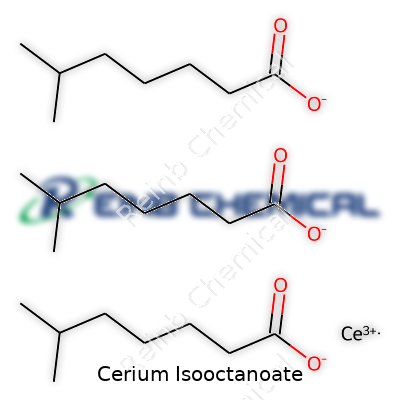
Cerium Isooctanoate steps quietly onto the stage, finding applications in areas like the plastics industry and catalysis. Its formula, C24H45CeO6, can sound intimidating at first glance, but each part of this formula tells a story. With cerium anchoring the molecule and isooctanoate groups shaping its chemical personality, this compound brings together a rare earth element and an eight-carbon acid. Each molecule contains one cerium atom and three isooctanoate (also called 2-ethylhexanoate) groups, creating a coordination compound tailored for specific reactivity.
In labs and factories, things rarely stick to neat textbook models. Cerium Isooctanoate becomes part of the toolbox because it brings together solubility and the gentle redox activity that cerium is known for. By design, three isooctanoate ligands stabilize the cerium, making it soluble in organic solvents. That opens doors for use as a plastic stabilizer and a catalyst for oxidative processes. I remember working on polymer additives and seeing metal carboxylates, including cerium, tweak the life span and color retention of materials without a fuss.
Safety becomes a concern as well. Cerium compounds get used in small doses, but an understanding of their makeup ensures environmental responsibility. Although cerium sits among rare earth metals, its carboxylate derivatives don't always behave the same way as the pure metal or basic salts. Knowing what’s in your compound goes beyond trivia—it’s part of managing risk and complying with regulations. The detailed formula tells you how much cerium you’re actually handling and guides hazard assessment.
Incorrect labeling or a mistaken formula can shut down an entire production line. During one project, an error in the listed formula of a catalyst led to dosing mistakes that affected both reactivity and waste output. With cerium isooctanoate, clarity around C24H45CeO6 means anyone picking up the bottle knows exactly the ratios involved. The industry needs this level of information for compliance, especially as environmental controls tighten internationally. Europe’s REACH and similar frameworks require declaration of chemical composition, down to the smallest detail.
Chemistry shapes sustainability efforts. Cerium isooctanoate’s main claim to fame lies in supporting cleaner industrial processes, especially as a replacement for more hazardous metal catalysts. The specific formula enables safer handling, but the road does not end there. Researchers have started exploring bio-based feedstocks for the isooctanoate portion, hoping to reduce reliance on petrochemicals in synthesis. Improving recycling and recovery methods for rare earths from spent catalysts also builds a longer-term vision.
Demand for cerium-based compounds likely continues to rise, shaped by the push for cleaner automotive, electronics, and plastics manufacturing. At the core sits the correct formula—C24H45CeO6. Every chemist, engineer, and policymaker involved in the supply chain relies on accuracy here, not just out of habit but as a real necessity. Getting it right keeps production safe, processes effective, and innovation possible, all while slowly pushing the industry toward greener, more responsible ways of working.
Most folks won’t bump into cerium isooctanoate unless they work in chemical labs or certain factories. In many coatings or polish-making shops, this stuff sits on the shelf as an additive that helps produce a smooth finish or boost performance. Folks around paints and specialty chemicals have probably seen it. On paper, cerium compounds often don’t ring alarm bells like mercury or lead, but that’s not a green light for carelessness.
Breathing in fine powders always raises a red flag at work. Dust or fumes—even from so-called “mild” compounds—put lungs at risk. Some reports show that certain cerium compounds, when handled carelessly or left to float freely in the air, can trigger coughing, eye irritation, and skin discomfort. Cerium isooctanoate belongs to a family of metal carboxylates. These don’t move through the body quickly, and repeated exposure can build up slowly, which heightens worry about chronic impacts.
Mixing or pouring liquids opens a more direct path to harm. Splashes to bare skin or eyes add real risk. The isooctanoate part means this isn’t just a metal salt; it’s paired with an organic acid that can boost absorption through skin. There’s no drama about acute poisoning in the news, but contact dermatitis and respiratory aches do appear in safety data sheets and case reports.
Reading through a safety data sheet, the language feels dry, yet warnings stand out: recommend gloves, goggles, and careful ventilation. OSHA and European agencies flag most rare earth compounds if mishandled, classifying them as “irritants.” There’s little research out there comparing toxicity levels, but similar compounds have shown they’re no friend to the kidneys or lungs after repeated dosing.
Cerium itself doesn’t trigger significant cancer worries based on animal tests. The issue rests in the chronic sniffing, splashing, or failing to clean spills, which happens more often than anyone likes to admit. Old-timers might brush off the warnings, yet new regulations treat rare earth compounds as an unknown needing respect.
Having worked around plenty of powdered metals and additives, experience taught me it’s wise never to trust a label just because it sounds mild. Relying on “safe enough” usually drives up incidents. Shops use local exhaust systems, gloves, and eye shields every single day, even when the boss grumbles about the cost. Training new hires on full wash-up protocols, spill response, and safe waste handling slows down work for a reason—one accident can cut a whole crew out of action.
I’ve seen folks pick up rashes or hacking coughs that last weeks, even with small exposures. Proper labeling and double-checking the shelf life of any chemical keep things safer. Disposing of leftovers in sealed drums, avoiding water drains, and sending waste to licensed handlers stops contamination outside the plant.
Cerium isooctanoate earns its keep in industrial settings. Respecting its risks, not rolling the dice on “it should be fine,” makes the work safer for everyone in the shop. Staying current on fresh data, listening to safety briefings, and treating every chemical as one step away from causing trouble—that’s what experience teaches, and it’s what keeps hands, lungs, and jobs safe in the long run.
Walking into a chemical storeroom, sometimes you get a feeling about what’s been handled with care and what’s been tossed onto a shelf. Cerium isooctanoate isn’t something you want to treat casually. This substance often shows up in high-tech fields — think catalysts, coatings, and specialty polymers. It has a respected job for a reason, but the only way to get its value is by storing it with the right attention.
Nobody wants to find out their chemical stocks have gone bad. Every chemist knows that moisture and oxygen can ruin many organometallics over time, turning something valuable into a useless sludge. Cerium isooctanoate has the same vulnerability. Leaving the container open for a minute or two may not mean much today, but give it a year, and the change adds up. Oxygen and water can speed up decomposition, changing not just the purity but sometimes causing the material to become sticky, discolored, or completely inactive for its original purpose.
Sticking cerium isooctanoate in just any jar won’t cut it. The best results come from airtight, chemical-resistant containers — glass usually works well, but sometimes high-grade plastics hold up just as well when glass becomes a hazard. Always make sure seals are clean and not cracked. Even a small leak eventually lets enough air and moisture in to ruin the lot.
Many mistakes start with storage close to a window or a heat source. Both sunlight and heat speed up unwanted reactions, including oxidation or even slow breakdown of the organometallic structures. I learned early on to pick shaded shelves, or better, sealed cabinets that never get warm. Regular room temperature — anywhere between 15°C and 25°C — usually works fine as long as temperatures stay steady without swings.
Someone who takes an extra minute to update storage logs avoids headaches later. If a container of cerium isooctanoate comes in with a certificate and production date, write that down and track each time the jar gets opened. Tracking storage duration pushes people to use up older stock, and less material gets wasted. It also helps identify any patterns if you notice changes in material over time, so bad batches get caught quickly.
Unmarked or poorly marked bottles get lost, ignored, or handled by mistake. Strong, simple labeling in large, easy-to-read print makes sure there’s no confusion, even for someone new on the shift. Add hazard warnings, handling tips, and the supplier’s information, and mistakes get cut down dramatically.
Chemicals rarely get ruined by just one mistake; problems stack up. New workers benefit from hands-on walkthroughs — there’s no substitute for watching someone who cares about both the material and the team. Ongoing training keeps everyone sharp about new risks, supplier changes, and any tweak in company policy. If everyone realizes that mishandled chemical stock impacts safety and project costs, there’s more buy-in to do it right.
Cerium isooctanoate holds its quality the longest in clean, dry, cool spaces with airtight containers. Careful labeling, regular audits, and a culture that respects good handling prevent headaches down the road. Tracking every batch and opening can catch small problems before they turn huge. For any specialized use, always check the safety data sheet and let manufacturers’ advice guide decisions about shelf life and disposable methods. These simple habits don’t just protect this valuable compound; they keep people and projects safe.
Cerium Isooctanoate seems like one of those specialty compounds people only hear about in an advanced chemistry class. But spend a bit of time in coatings, plastics, or fuel industries and its role becomes obvious. I first came across cerium carboxylates when volunteering for a small research lab that supported a local automotive plant. Researchers there looked for ways to boost the toughness and stability of paint jobs—an ongoing battle. Here’s where the story of Cerium Isooctanoate gets interesting.
Walk into a car showroom filled with glossy sedans, and you’ll see the benefit of good surface protection in action. Cerium Isooctanoate works as a top-drawer drier and surface modifier, especially in alkyd and polyester coatings. Paint formulators often face trouble with slow curing times or colors fading on sunlit surfaces. By introducing cerium salts, manufacturers get quicker curing and a finish that stands up better to light or atmospheric attack.
The science points to cerium’s unique oxidation properties. It limits yellowing and chalking in clear and colored finishes. That translates to less maintenance and fewer complaints from car owners or furniture makers. Industry data supports these claims, showing greater resistance to weather and improved gloss retention with cerium-based additives. I’ve seen cross-sections of treated panels after extended outdoor testing—paints with Cerium Isooctanoate keep their shine significantly longer than untreated ones.
Though coatings get a lot of attention, plastics benefit too. Cerium Isooctanoate enters the mix during polymer stabilization. Polypropylene, for example, can suffer from sunlight. Cerium-based additives absorb harmful rays and slow down the slow breakdown process called photo-oxidation. Some reports show as much as 30% longer product life for outdoor plastic goods that receive this treatment.
Rubber compounding uses cerium salts in smaller doses to enhance cross-linking, making tougher seals and hoses for automotive or industrial use. Working with a friend who installs irrigation systems, I saw how hoses treated with these additives survived for years under harsh sun and pressure fluctuations. These are the unseen battles that keep water flowing and machines running.
Fuel additives make or break efficiency and pollution. Cerium Isooctanoate acts as a combustion promoter in both gasoline and diesel. Engines burn cleaner with smaller particles of carbon in their exhaust, cutting down on damaging soot. A handful of mining trucks that switched to cerium-treated diesel showed cleaner filters and fewer emissions—they passed stricter checks with ease. The National Renewable Energy Lab points out that cerium-based additives can cut soot by over 20% compared to untreated fuel.
Using Cerium Isooctanoate by itself doesn’t guarantee success. Manufacturers keep a close eye on dosage to avoid overuse and monitor for any side effects in air or water runoff. Companies also look for sources that follow good practices for rare earth extraction and refining. People expect both durability and environmental responsibility these days, and experts are working on better recycling and waste disposal methods.
Cerium Isooctanoate shows how something obscure in a textbook drives real-world advances. From vibrant car paints to cleaner fuels and long-lasting plastics, this compound keeps production lines moving and products lasting longer. Customers end up with better-performing goods, while industries try to push these benefits further with smarter chemistry and more responsible sourcing.
| Names | |
| Preferred IUPAC name | Cerium 3,5,5-trimethylhexanoate |
| Other names |
Cerium(III) 2-ethylhexanoate Cerium Octoate Cerium 2-Ethylhexanoate Cerium(III) Isooctanoate |
| Pronunciation | /ˈsɪəriəm aɪˌsəʊˈɒk.təˌneɪ.ət/ |
| Identifiers | |
| CAS Number | 2081-61-6 |
| Beilstein Reference | 3958776 |
| ChEBI | CHEBI:131982 |
| ChEMBL | CHEMBL4296980 |
| ChemSpider | 19771759 |
| DrugBank | DB11155 |
| ECHA InfoCard | 100.244.346 |
| EC Number | 3105-77-3 |
| Gmelin Reference | 143697 |
| KEGG | C18386 |
| MeSH | D002533 |
| PubChem CID | 159789697 |
| RTECS number | RR3679000 |
| UNII | 139255411A |
| UN number | UN3082 |
| CompTox Dashboard (EPA) | Q107086925 |
| Properties | |
| Chemical formula | C24H45CeO6 |
| Molar mass | 570.98 g/mol |
| Appearance | Light yellow transparent liquid |
| Odor | Slight characteristic |
| Density | Density: 0.95 g/cm³ |
| Solubility in water | Insoluble |
| log P | 3.8 |
| Basicity (pKb) | pKb: 5.7 |
| Refractive index (nD) | 1.520 |
| Viscosity | 50 - 300 mPa.s |
| Dipole moment | 1.17 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 395.8 J·mol⁻¹·K⁻¹ |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Precautionary statements | P261, P280, P305+P351+P338, P310, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-1-0 |
| Flash point | Flash point: >110°C |
| LD50 (median dose) | LD50 (median dose): >5000 mg/kg (rat, oral) |
| PEL (Permissible) | 10 mg/m3 |
| REL (Recommended) | REL (Recommended Exposure Limit) of Cerium Isooctanoate is: "5 mg/m³ (as Ce) |
| Related compounds | |
| Related compounds |
Cerium neodecanoate Cerium octoate Cerium(III) 2-ethylhexanoate Cerium carboxylates Lanthanum isooctanoate |