
Manganese isooctanoate’s roots stretch back to the rise of organometallic compounds in the twentieth century, a period when chemistry research charged ahead to meet growing industrial demands. The journey began with the hunt for stable manganese compounds to improve performance in coatings, lubricants, and polymers. Old-school salts and oxides offered their own benefits, but researchers kept searching for a blend of stability, solubility, and reactivity. Chemical engineers in the late 1960s discovered a niche for manganese carboxylates, with isooctanoate showing standout traits. Once the practical value of metal soaps hit the paint and coatings sector, manganese isooctanoate found itself increasingly in demand, often tied to the greater push for efficient driers and improved polymer processes. Over fifty years, tweaks in synthesis, purification, and safety knowledge shaped the product people use today. Big chemical names and university labs both played roles in mapping out best practices, standardizing qualities, and characterizing performance for global industry.
Manganese isooctanoate stands out as a metal-organic salt where manganese meets isooctanoic acid. Chemically speaking, this compound rides a fine balance: manganese brings its oxidizing muscle, while the organic tail makes it oil-soluble and easier to blend with resins and other oils. Formulators prefer it because it dissolves in organic solvents and stays stable under typical manufacturing conditions. Every batch, if handled right, delivers a clear, amber to brownish liquid or occasionally a soft solid, depending on synthesis tweaks. Industries use it as an oxidative drier, coaxing paints, inks, and coatings into quick, even curing. Off the shelf, companies find it as a solution in hydrocarbon solvents, flagged with active metal content to simplify formulation work.
In lab settings, manganese isooctanoate comes in several concentrations, often between 4% and 12% manganese by weight. Its density hovers around 0.95–1.05 g/cm3 at room temperature. Some batches swing slightly yellow, others take on a deep brown tint, mainly due to differences in raw acid, trace impurities, or aging. The scent is mild but unmistakably organic. Its oil solubility marks a major plus; water has little effect, but the compound goes easily into aliphatic and aromatic hydrocarbon solvents. Chemically, it reacts in air, especially in the presence of unsaturated fats and resins, speeding up polymerization—a reason why it excels in curing oils and alkyds. Unlike dry powders, this solution-form product resists caking and is easy to blend, with flash points just north of 50°C, asking for mindful storage.
Product datasheets from leading manufacturers spell out more than just manganese content. Labels flag solvent type, acid value, active metal concentration, and recommended storage temperature. Companies often run quality checks for water content, residual acidity, and heavy metals, keeping impurities below industry thresholds. For example, technical grades destined for paints require <2% water and as little lead as possible—often less than 0.01%, thanks to tightened regulatory oversight in the European Union and North America. Standard packaging comes in steel drums or HDPE containers, with labeling strictly following GHS/REACH guidelines. Transporters stamp containers with UN numbers, proper shipping names, and hazard icons, helping everyone in the supply chain know what they’re handling.
Making manganese isooctanoate starts with reacting manganese dioxide or manganese carbonate with isooctanoic acid, usually in the presence of an oxidizer. It takes some heat and careful stirring to move from a mass of powder to a clear salt. Chemical engineers watch temperature closely; too hot and the product breaks down, too cold and the reaction stalls. To get the right concentration, manufacturers pull vacuum or sparge with nitrogen, driving off water and bumping up purity. Once formed, the mixture passes through filters to strip away free manganese compounds or leftover acid. Some operations go even further, using solvent extractions or column purification to clean up the salt, all to deliver a product free of grit or sludge. Particle size, viscosity, and color all get checked batch by batch—a headache for engineers, but critical for high-spec paints and inks. Final solutions mix the salt into a hydrocarbon base, except for odd specialty orders.
Manganese isooctanoate serves as an active catalyst in chemical systems where oxygen absorption speeds up crosslinking. Put it in contact with alkyd resins or linseed oil, and the manganese atom swings between oxidation states, nudging the oxygen around and setting off the polymerization process. This ability gets fine-tuned by blending with other metal carboxylates—cobalt or zirconium compounds, for example—which shape the overall drying curve or sidestep yellowing. Tweaks in isooctanoic acid chain length or branching can slightly change how the molecule wet surfaces or dissolve in solvents. Over the years, teams studying this compound found that subtle changes in synthesis, such as extended reaction times or purifications, shave down unwanted by-products that might trip up curing or color performance.
Chemists may call manganese isooctanoate by other names: manganese 2-ethylhexanoate, manganese(II) isooctanoic acid salt, or even manganese octoate. These labels pop up across datasheets, trade documents, and regulatory filings. Some suppliers use brand names, trading under proprietary or abbreviated tags, but the backbone stays the same—a manganese carboxylate based on branched C8 acid. Set aside the brand differences; chemical ID and safety data sheets spell out the active ingredient, helping buyers confirm what lands in their drums.
Nobody cuts corners with safety on manganese isooctanoate. In my years around paint labs and chemical warehouses, every job starts with gloves, goggles, and proper ventilation. In liquid form, it slips through gloves fast, so skin contact means a trip to the eye-wash, and the organic solvents demand good air handling. Inhalation over long stretches causes headaches or worse, so teams use closed systems or local exhausts at the mixing station. European legislation, North American EPA rules, and China’s growing regulatory system all outline tight exposure limits for manganese compounds because long-term inhalation and skin uptake can lead to nervous system issues. Spill kits wait nearby, and workers store drums in well-ventilated, cool sheds, away from direct sun. Safety data sheets lay out fire precautions; the solvent base means every facility posts extinguisher locations and emergency shutoff details.
Paint and coatings manufacturers lean on manganese isooctanoate for its role in drying and curing alkyds. Where older formulas relied on cobalt, concerns about toxicity and metal scarcity have nudged many companies towards manganese-based systems. Adding it to varnishes, artists’ oils, or industrial enamels, formulators notice sharper, more reliable cure cycles—even when ambient humidity swells or temperatures drop. that reliability cuts down on production snags, speeding up line runs and reducing the need for expensive reworks. Polymer and plastic engineers reach for manganese isooctanoate when they need controlled oxidation in manufacturing, especially during the toughening of polyester and polyurethane resins. Grease makers also work it in as a curing aid, giving lubricants their special performance at high-temperatures and heavy loads. Across different applications, people thirst for fast, even, and safe films, and manganese isooctanoate continues to answer that call, especially where regulatory needs keep evolving.
Research in university and industrial labs keeps pushing the frontiers on manganese isooctanoate. New projects aim to swap out old solvent carriers for greener, less volatile alternatives. Others focus on making low-manganese blends that balance cure speed with eco-friendliness, answering pressures from customers and regulatory bodies alike. Scientists use high-throughput screening to test tweaks in acid component or purity—chasing longer shelf life or boosted performance in tough humidity. Digital tools, like molecular modeling, now speed up the hunt for better blends and predict how tweaking the molecule might dial performance up or down. Industry partnerships with academic chemists spawn patents around the world, with new methods to stabilize formulation, reduce manganese leaching, and make recycling of drying agents more viable.
Toxicologists take manganese exposure seriously, whether in the form of dusts or oil-bound salts. Old studies linked inhalation of manganese at industrial levels with tremors, coordination loss, and subtle cognitive impacts—a reminder that workers in drying, paint, and polymer plants need robust protection even from liquid products. More recent work tests environmental effects after disposal or spill. Wastewater studies show that isooctanoate itself breaks down faster than other long-chain carboxylates, but the manganese atom might persist, especially in soils and riverbeds close to chemical factories. Researchers keep looking for ways to block manganese build-up in the food chain and test new purification or neutralization steps during production. Regulatory agencies react by updating exposure limits and flagging manganese isooctanoate for close tracking, forcing manufacturers to report usage and, in some cases, switch to lower concentration blends.
Trends point toward growing demand for manganese isooctanoate, fueled by regulations on cobalt, the climb in waterborne and VOC-free coatings, and new polymer technologies. Research into safer metal carboxylates has the potential to push the market forward, with manganese compounds that match or outdo rivals on speed and reliability. If green chemistry keeps getting traction, future synthesis will move away from harsh solvents and look for bio-based acids as starting points, shrinking the carbon and toxicity footprints. Customers also look for digital traceability and lifecycle labels—tracking every batch from raw material to finished product. As industry shifts, continued investment in worker safety, product purity, and application testing will help manganese isooctanoate stay useful in the years ahead, driving both creativity and responsibility further down the supply chain.
Manganese Isooctanoate isn’t the kind of chemical that finds its way into everyday conversation, but it plays a big part behind the scenes in industries that most folks rely on, even if they don’t know it. At its core, it's a metal carboxylate—basically manganese attached to an organic acid. Most of the buzz around manganese isooctanoate comes from its use as a drying agent, also known as a drier or siccative, in paints, coatings, and inks.
Ask anyone who has spent time painting a room: nobody enjoys waiting for paint to dry. Companies use manganese isooctanoate to nudge that process along. Thanks to its unique makeup, it helps oxidize and solidify the oils in paint, speeding up the drying process. This might sound technical, but for both industrial manufacturers and regular folks, it simply means a job finishes quicker and with fewer problems like dust sticking to wet layers.
Manganese-based driers also step in when legislation or environmental rules tighten restrictions on heavy metals like lead and cobalt. Less toxic than these heavy metals, manganese is often safer for workers and the environment, which matters in settings where large volumes of coatings get applied daily. According to the European Chemicals Agency, manganese substitutes have become more common as countries clamp down on harmful substances that put both water sources and people at risk.
Some might be surprised, but this compound gets mixed into printing inks—especially those used in packaging. Let's say you've picked up a box of cereal with sharp graphics and bold colors. Fast-drying inks keep those colors distinct, prevent smudging, and help the packages move smoothly through the production floor. Modern logistics can’t afford holdups, so the chemistry inside manganese isooctanoate helps keep goods moving.
It makes an appearance in plastics and rubber manufacturing. In those fields, the compound can kickstart polymerization reactions, which means raw materials turn into finished goods without delays. This can spell the difference between a smooth production run and a costly delay. A chemist once told me that a slight hiccup in drying or curing stages of manufacturing can cost thousands, or in some cases, wipe out a week’s profit.
The flip side of every industrial wonder is its impact beyond the factory fence. While manganese compounds rank ahead of the old lead driers for safety, no chemical gets a total pass. Inhalation can irritate, and there’s always the bigger-picture worry about chronic exposure. Responsible manufacturers now keep close tabs on workplace ventilation and personal protective equipment. Reputable suppliers provide certificates of analysis, batch testing, and safe handling paperwork. This helps customers verify claims—a big component of Google’s E-E-A-T principles about expertise and trustworthiness.
On the environmental front, manganese doesn’t stick around in the same way as heavy metals. That’s good news for lakes and soil—but only if waste and rinse water get handled properly. This is where more companies should invest in high-efficiency recovery systems instead of letting process water leave the site untreated. Even a small step, like training workers to recognize spill risks, can prevent unnecessary releases.
If there’s a lesson here, it’s that chemistry keeps evolving. A decade ago, hardly anyone asked what chemicals dried their paint or ink, now, customers and regulators expect transparency. Manufacturers that test for low-toxicity options, train their teams, and give honest information tend to earn respect on both the shop floor and the consumer end.
Manganese isooctanoate represents this shift. It takes the load off legacy chemicals and points the way toward safer, faster, and more reliable products. The key is keeping up with new research, being honest about risks, and choosing chemistry that respects both people and the planet.
Whenever people bring up Manganese Isooctanoate, the talk usually skips straight to uses and skips the gritty details. But the real insight lives in understanding what’s actually in the compound. Manganese Isooctanoate isn’t complicated once you know the basics. Every time you see a bottle labeled “manganese neodecanoate” or “manganese 2-ethylhexanoate,” it’s usually the same thing—different names pointing at the same molecule. That molecule starts with the 2-ethylhexanoate anion paired with a manganese atom.
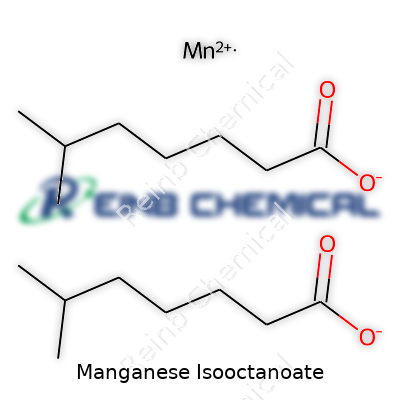
Manganese itself is a transition metal. On its own, it's not much use for coatings or drying oils. When it hooks up with an organic acid like isooctanoic acid (also called 2-ethylhexanoic acid), the structure changes: now you have a carboxylate group instead of a simple manganese salt. The most common version used in paints or lubricants comes as manganese(II) 2-ethylhexanoate; the manganese ends up with two carboxylate ligands clinging to it.
The backbone here is simple chemistry. 2-Ethylhexanoic acid carries the formula C8H16O2. Losing a hydrogen to become an anion pushes it to C8H15O2–. Two of those anions gather around a manganese(II) ion (Mn2+), making the overall formula: Mn(C8H15O2)2. In plain words, it’s manganese stuck between two organic tails.
That structure explains why this compound works so well in catalysis and coatings—the manganese atom gets delivered in a form that dissolves well in organic systems, spreading easily into oil-based varnishes or alkyd paints. Many folks in the automotive or construction world see it in metal driers, not realizing that neat pairing of metal with a fat-soluble ligand is what makes it so useful.
The value of spelling out the formula sits in both safety and performance. Knowing it's Mn(C8H15O2)2 helps a formulator predict how the compound will break down, how it might react with other additives, and why it behaves differently from simple manganese salts. Out in the lab, I’ve seen people guess at additives, hoping to fix a drying issue, only to scramble when something doesn’t jive—most of that confusion comes from not understanding what really goes into the formulation. A metal carboxylate like this brings different hazards and different regulatory status than raw manganese—so the details matter in compliance checks and material safety sheets.
Every time we handle these compounds, experience tells us clear info cuts down on both guesswork and accidents. It matters to manufacturers, sure, but it matters just as much on the shop floor, where people are blending chemicals by the drum. One missing hydrogen or a stray metal can change flammability or toxicity.
More and more companies are drawing maps for their teams—putting up posters, running hands-on workshops, and sharing easy-to-read cheat sheets for tricky formulas. Digital tools now exist to break down formulas step by step, connecting the dots in a way that even those without a chemistry degree can follow. Regulatory bodies suggest moving toward clearer labelling: not just the name, but the formula spelled out right on the packaging. The less room for mix-ups, the safer and more effective our products turn out.
From drying paints to rubber processing, Manganese Isooctanoate crops up in several industries. It’s not as famous as some chemicals, but it still deserves respect. I’ve seen what happens when folks overlook small safety details, and it’s rarely pretty. Headaches, skin rashes, or unreliable equipment can follow not paying enough attention. This compound isn’t wildly volatile, yet trouble follows careless stashing or loose habits.
Dusty corners or cluttered shelves tempt disaster. To store Manganese Isooctanoate with confidence, aim for rooms under 25°C, away from sunshine, furnaces, or open flames. Heat shortens shelf life and kicks off unwanted reactions, giving you headaches down the road. Most spills I’ve seen grew from overstuffed storerooms, cracked bottles exposed to warmth, or stingy air-conditioning.
Leave humidity out of the picture. Dampness never helps; moisture can gum up the product and make containers sweat. Moist or leaky storage leads to lumps and, in a worst-case scenario, chemical breakdown. Expensive, ugly messes often start simple—avoid ruined stock by keeping things dry and sealed.
Any container holding this compound should close tightly. If the cap wiggles, swap it. Stirring up vapors in a stuffy room can irritate the nose and throat and might lead to lightheadedness. This happens mostly because someone forgot to tighten up a drum or left a lid off overnight.
Don’t skimp on correct labels. I’ve mixed up bottles before, thinking I’d remember which was which. That shortcut turned a dull afternoon into a scramble for eyewash and MSDS sheets. Clear labeling saves stress and prevents accidents, especially when several products look similar.
Keep any spark sources far from storage. Static discharge could ignite some metal soaps, and that’s never an easy cleanup. Think through the wiring, keep tools grounded, and make sure outlets are inspected. Even smoke breaks outside can lead to carelessness if the habits get loose.
Use proper drums, not makeshift containers. Some metals in barrels react poorly, especially with time. Stick with polyethylene or compatible plastics. Metal drums aren’t always a fit—corrosion can kick off long-term headaches, leaving you with leaks nobody noticed until it’s too late.
Wear gloves and splash goggles, even during a quick transfer. Manganese compounds can cause skin irritation if ignored. I’ve learned that direct contact—however brief—leaves you itching and regretting laziness. Make sure there’s always an eyewash station within reach.
A clear spill plan in the workplace lowers the risk. Use absorbent sand or recognized materials for cleanup. Never let it run down drains—environmental fines follow fast. Ventilate the area after a spill to clear fumes, and use approved waste containers. Your future self (and your coworkers) will thank you.
Routine matters just as much as reaction time. A basic checklist for storage checks, scheduled inspections, and personal protective gear makes all the difference. I’ve seen more than a few teams turn close calls into teachable moments because they kept things organized.
Handling Manganese Isooctanoate never rewards shortcuts—protective gear, proper storage, and awareness do more than meet legal standards; they build a safer workplace, keep people healthy, and protect the bottom line. Just tightening a lid or closing a valve goes farther than most realize.
Manganese isooctanoate shows up in places many folks never consider—catalysts for coatings, driers in paints, and stabilizers in some plastics. It’s not a headline chemical since most eyes go to lead, mercury, or more infamous toxins. Still, a closer look tells us it deserves some respect, especially when it comes to how it sits in workplaces and products.
If you pour concrete or work with alkyd paints, you probably know the tang of solvent fumes and the dust that sticks to your skin at the end of a shift. Manganese isooctanoate gets handled mostly by trained staff in industry, so public exposure—unless you’re in the thick of manufacturing—tends to stay limited. The larger concern isn’t whether it’s at a hardware store, but how workers interact with it, and what’s left behind in products.
Breathing in high levels of manganese dust or fumes never served anyone well. Science points out that chronic inhalation can damage the nervous system. You might see symptoms in welders or folks in battery factories. Tremors, mood changes, and slow movement sometimes show up after years of exposure. Manganese isooctanoate isn't as volatile as pure manganese dust, but if it’s disturbed, mist or vapor can still lead to risk.
Rules exist for exposure: Both OSHA in the U.S. and the European Chemicals Agency put specific limits on workplace air. Employers must use ventilation, respirators, and train staff to wash up and not eat on the job. Yet, accidents happen, and standards on paper do not always match real-world practice. A small shop might cut corners or run older extraction systems, putting workers closer to the line.
I’ve worked around industrial chemicals long enough to respect how easily safety drifts out of mind. A tight deadline, a broken exhaust fan, or a shipment delayed can tempt even careful staff to get the job done and overlook masks or gloves. While manganese isooctanoate might not leap off the hazard charts like some others, it brings harm if routine protections aren’t in place.
Anything lost down the drain or sent up a flue stack doesn’t disappear. Soils near manufacturing plants sometimes test high in manganese, affecting crops and water supplies. Some regions in Brazil and India, with loose industrial oversight, present stories of children with learning difficulties linked to heavy metal runoff, manganese among them. It doesn’t mean every gallon of product is a toxic threat, but accumulation—especially unchecked—starts trouble over years.
Some companies push for alternatives with less risk, especially where workers’ health drew too many questions. More transparent labels, better training, and faster adoption of less hazardous driers make a difference. Simple fixes work: good ventilation, regular air monitoring, and routine health checks. Governments could fast-track reviews of safe threshold levels as research uncovers new evidence.
Reading the label, asking questions at the local paint shop, and supporting brands with better safety records doesn’t just check a box. It sets pressure upstream for safer workplaces and communities. Manganese isooctanoate matters because folks in overalls and gloves matter. No product justifies risking health behind a warehouse door.
Manganese Isooctanoate always shows up in conversations about improving coatings, inks, or drying oils. The chemistry behind it draws from the way manganese ions kick off the drying process in alkyd resins and paints. These industries usually tap into concentrations between 6% and 12% manganese metal by weight. Formulators tend to use final product dosages ranging from 0.01% up to 0.2% metal content, depending on what they're trying to achieve. Getting the dosage right is not about hitting an arbitrary mark. It means finding a sweet spot that provides effective drying without triggering side reactions or stability problems.
You might wonder why companies pick 6%, 8%, or maybe 10% manganese. The answer traces back to how much active manganese the process needs to start the catalytic reaction. At lower concentrations, drying slows down, leaving surfaces tacky longer than anyone wants. Bump up the content too high, and you face problems like over-drying, which leads to wrinkling or brittleness. Some paint techs told me they always watch out for a “skin” forming on top of the can, a classic sign of too much drier. Cost matters too. Manganese is a specialty ingredient, so companies want to add just enough—no more, no less.
Using manganese compounds calls for care, since exposure to high levels can affect the nervous system. All reputable manufacturers pay attention to the workplace exposure guidelines from groups like OSHA and the ACGIH. Keeping levels in that 6-12% manganese range lets handlers avoid excessive dust or vapor during production. I remember every shop I visited, the foreman reminded new hires to use gloves and proper ventilation even if the percentage sounded small. Safety isn’t just a rule; it’s what keeps workers coming back day after day.
Environmental rules grow stricter every year. The low concentration approach isn’t only about saving money—it helps meet emissions limits and waste targets. Companies switching from cobalt driers (which raise more red flags around toxicity) to manganese isoctanoate say they appreciate the lower hazard profile, both for employees and the environment. By keeping the concentration precise, they can deliver the drying power without racking up disposal headaches.
Some manufacturers go beyond routine dosing. They lean on automated dosing systems to mix the drier exactly right, limiting human error. Others work with additive suppliers who offer pre-measured packs calibrated to the size of a production batch. These steps guard against slips that could cost time or trigger health risks. As people share data and learn from experience, best practices spread quickly through conferences and technical bulletins.
Dosing isn’t just about numbers on a label. A small miscalculation can slow production or create unpleasant surprises in the final product. I’ve seen engineers who test limited-run batches before full production just to avoid these headaches. With manganese isoctanoate, the industry’s collective knowledge helps keep dosages squarely in that 6-12% manganese content window. This approach balances performance, safety, and cost—a formula that works as well in the mixing tank as it does on the finished surface.
| Names | |
| Preferred IUPAC name | manganese 2-ethylhexanoate |
| Other names |
Manganese 2-ethylhexanoate Manganese octanoate Manganese(II) isooctanoate Manganese(II) 2-ethylhexanoate |
| Pronunciation | /ˌmæŋ.ɡəˈniːz aɪ.səˌɒk.təˈneɪ.ət/ |
| Identifiers | |
| CAS Number | 14375-98-5 |
| Beilstein Reference | 3858730 |
| ChEBI | CHEBI:91207 |
| ChEMBL | CHEMBL612206 |
| ChemSpider | 14234978 |
| DrugBank | DB11473 |
| ECHA InfoCard | 07c84deb-bcab-4a6e-96ed-c4d7b1f4d479 |
| EC Number | 284-938-8 |
| Gmelin Reference | Gmelin Reference: **1152** |
| KEGG | C18659 |
| MeSH | Manganese Compounds |
| PubChem CID | 159232 |
| RTECS number | OGG83510MM |
| UNII | 39738HN1QP |
| UN number | UN3082 |
| Properties | |
| Chemical formula | C16H30MnO4 |
| Molar mass | 445.56 g/mol |
| Appearance | Dark brown clear liquid |
| Odor | Odorless |
| Density | 0.98 g/cm³ |
| Solubility in water | Insoluble in water |
| log P | 3.8 |
| Vapor pressure | Negligible |
| Basicity (pKb) | 9.41 |
| Magnetic susceptibility (χ) | +4940e-6 |
| Refractive index (nD) | 1.4950 |
| Viscosity | Viscosity: 63.6 mm²/s (40°C) |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 186.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | A12CC04 |
| Hazards | |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P271, P273, P280, P303+P361+P353, P305+P351+P338, P312, P370+P378, P403+P235, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | > 64°C (147°F) |
| Lethal dose or concentration | Lethal dose or concentration: LD₅₀ Oral Rat > 5000 mg/kg |
| LD50 (median dose) | > 2,000 mg/kg (rat, oral) |
| NIOSH | Manganese Isooctanoate does not have a specific NIOSH number assigned. |
| PEL (Permissible) | 5 mg/m3 |
| REL (Recommended) | 500 - 2000 mg/kg |
| IDLH (Immediate danger) | IDLH not established |
| Related compounds | |
| Related compounds |
Manganese(II) acetate Manganese(II) oxide Manganese(III) acetylacetonate Manganese naphthenate Manganese stearate |