
Back in the 20th century, as refining processes advanced, chemists began to recognize the potential of transition metal carboxylates for catalysis. Nickel isooctanoate entered industrial production during a time when the search for more effective chemical promoters intensified, especially in polymer and paint manufacturing. Early work explored organic acid complexes that improved stability and dispersion, leading manufacturers to focus on mid-chain fatty acids like isooctanoic acid. Over decades, the use shifted from simple laboratory reagents to specialized catalysts with consistent performance. By the 1980s, broad industrial adoption had started, providing a foundation for further chemical innovation, including improved environmental standards for catalyst residues.
Nickel isooctanoate often appears as a greenish to bluish liquid, depending on concentration and purity. This complex serves primarily as a catalyst or curing agent in coatings, plastics, and polymerization reactions. It forms through the reaction of nickel salts with isooctanoic acid, yielding a compound with good solubility in many organic solvents. Anyone working in the manufacturing sector or with specialty chemicals has likely encountered its distinctive coloration and mild, oily scent during handling. Producers have refined formulations over the years so that the product incorporates the stability and ease of use required for demanding applications.
Nickel isooctanoate stands out for its unique combination of solubility and reactivity. It generally remains stable under normal storage conditions and displays a relatively high boiling point, often in the 300–350°C range. Its molecular weight varies depending on hydration or ligand association but typically lands between 320 and 340 g/mol for the basic stoichiometric product. Density hovers around 1.0 to 1.1 g/cm³. This compound resists hydrolysis reasonably well, which helps maintain shelf-life, but still reacts with strong acids or bases. Chemically, it presents a balance between lipophilicity and metal center reactivity, making it ideal for organic reaction environments.
Manufacturers provide technical documentation that includes nickel content (usually 10–12% by mass), viscosity, and purity level for industrial lots. The color index leans toward green, a telltale sign of nickel ions in organic coordination. Safety labeling draws from GHS guidelines, with typical hazard classifications recognizing toxicity to aquatic life and potential health risks upon inhalation or ingestion. Labels identify batch number, net volume or mass, manufacturer’s contact details, recommended storage temperature, and expiration date. More advanced suppliers now offer certificates of analysis certifying trace metal impurities, moisture content, and specific gravity—an industry response to higher regulatory expectations.
Production involves neutralizing nickel(II) salts, such as nickel carbonate or nickel chloride, with 2-ethylhexanoic acid in a suitable solvent—often an alcohol or aromatic hydrocarbon—to ensure efficient reaction and subsequent extraction. The process moves through mixing, agitation, and controlled heating, followed by filtration and vacuum drying to remove unreacted species and volatile solvents. Sometimes, preparatory processes direct the stoichiometry toward slightly basic conditions to favor formation of the neutral nickel isooctanoate, minimizing leftover acid and other impurities, which could interfere with downstream catalysis work.
Within organic syntheses and industrial reactions, nickel isooctanoate acts as a competent catalyst, particularly in oxidation, polymerization, and crosslinking reactions. It enhances rates of autoxidation in alkyd paints, contributing to faster surface drying and film development. Chemists have experimented with ligand modification to tailor reactivity: substituting isooctanoate with similar carboxylate groups shifts solubility and performance. In catalysis, nickel’s ability to toggle between oxidation states drives innovation, especially as researchers challenge older catalytic systems to deliver higher selectivity and lower toxicity.
Nickel isooctanoate goes by several names—nickel 2-ethylhexanoate, nickel octanoate, and sometimes simply “nickel carboxylate.” Commercial sources may refer to trade names such as Niox, Nicocat, or specialty codes with a manufacturer’s suffix. These synonyms can create confusion across regional markets, so precise CAS numbers and chemical formulas now play a larger role in supply chain integrity. Anyone sourcing or using nickel isooctanoate regularly soon learns to check accompanying chemical identifiers rather than relying solely on a trade name.
Handling nickel isooctanoate brings occupational health into focus. The compound poses both acute and chronic risks, including respiratory irritation, dermatological reaction, and possible systemic toxicity with long exposure. Proper PPE—gloves, goggles, respirators—remains standard in any operation. OSHA and EU standards recommend specific exposure limits for nickel compounds. Industrial hygiene programs monitor air and surface contamination closely, combining containment levels with frequent risk assessment. Emergency protocols for spills and overexposure emphasize ventilation, prompt decontamination, and, if necessary, medical surveillance.
Nickel isooctanoate has carved out niches in surface coatings, plastics, chemical synthesis, and elastomer formulations. Paint manufacturers depend on its catalytic punch to accelerate drying without yellowing, while the polymer world taps nickel carboxylates for crosslinking specialty resins. In synthetic chemistry, reactions demanding homogeneous metal distribution lean on this compound’s solubility and reactivity. Use has shifted across decades—greener alternatives push companies to revisit catalyst loads, lower process emissions, or scan for residue in finished products. The electronics field finds value in precise nickel doping of functional polymers, linking the compound to emerging high-tech applications as well.
Research trends target safer, more selective, and less environmentally persistent nickel carboxylates. Recent academic projects explore structure-property relationships: how tweaking alkyl chain length or introducing functional groups at the acid moiety can produce superior catalysts. Other teams work on solventless or low-waste preparation strategies to address sustainability mandates coming from both government and large purchasers. Analytical chemists validate new techniques for residue tracking at parts-per-billion levels, while polymer scientists run side-by-side trials with alternative drying agents to map out performance gaps.
Toxicology studies confirm nickel compounds pose significant risk for skin sensitization and carcinogenicity potential, with much work focused on outlining permissible exposure levels. Inhalation studies, both in vivo and in vitro, point toward lung deposition and persistent inflammation, which moves regulators to revisit occupational standards. Environmental fate studies trace persistence and bioaccumulation. Risk assessments compare acute and chronic doses, often using mammalian cell cultures and animal models to simulate workplace exposure. The evidence highlights the need for stricter personal protection and the drive for less toxic alternatives in catalytic applications.
Demand tracks the push for faster, more efficient chemical processes, tempered by the greater weight given to toxicity and environmental stewardship. Catalysts like nickel isooctanoate stand under scrutiny as regulations tighten. Future directions lean into green chemistry—biodegradable ligands, lower-nickel loadings in formulations, and closed-loop recovery of spent catalyst solutions. Digitalization in the laboratory and plant promises better tracking of exposures and real-time safety controls, while startups look to challenge incumbents with innovative, less hazardous alternatives. Collaboration between chemical suppliers, manufacturers, and regulators will shape the next chapter, so those who adapt most nimbly to evolving expectations are positioned to lead.
Nickel isooctanoate might sound like something tucked away in a scientist’s supply closet, but its reach extends into products and processes most folks rely on every day. I’ve seen firsthand how specialty chemicals like this can shape the durability, efficiency, and even the safety of everyday materials. Understanding what nickel isooctanoate does and why it matters sheds light on some decisions that manufacturers face and those ripple effects that land on us as consumers.
Many paints, plastics, and coatings depend on alkyd resins for their strength and flexibility. Here’s where nickel isooctanoate steps in as a powerful catalyst. In the world of resin manufacture, controlling the rate at which resins cure or dry can be the difference between a sticky mess and a hardened, long-lasting surface. Nickel-based compounds help manage this process. It’s a behind-the-scenes player, but without it, the coatings on bridges, ships, and even buildings wouldn’t last as long or resist corrosion quite as well. Working around construction for years, I have seen the difference between a high-quality coating and a shortcut job. Cutting corners on catalysts can mean costly repairs later.
Modern polymers don’t thrive on their own; they often need stabilizers to fight off breakdown from sunlight, heat, or chemicals. Nickel isooctanoate is used as an additive to help plastics hold up under tough conditions. Think of it as giving certain plastics and rubbers a longer lease on life. While cycling through old automotive parts, I’ve noticed how rubber hoses treated with the right additives stand the test of time while untreated ones crack and fail. These additives, though not visible, play a key role in product reliability.
Manufacturers use nickel isooctanoate in the process of reformulating fuels to meet environmental regulations. This compound serves as a catalyst that helps break down impurities and improve burn characteristics. As emission standards have tightened, I’ve watched the fuel industry invest in every possible angle, from the refinery all the way to what comes out of a car’s tailpipe. Catalysts like nickel isooctanoate contribute to cleaner, more efficient fuels—a small part of the puzzle, but a crucial one for reducing pollution.
No chemical story feels complete unless we consider the environmental price tag. Nickel compounds, if mishandled, pose risks to health and ecosystems. Exposure during manufacturing or disposal becomes a problem without robust safeguards. I’ve met environmental engineers who stress traceability and recovery systems that make sure these materials don’t enter water supplies or get dumped unsafely. Using closed systems, proper training, and continuous monitoring offers a direct path to responsible use. The chemical industry has plenty of room to advance transparency and communication with the public so concerns can be addressed before becoming crises.
Nickel isooctanoate won’t make headlines, but its role in keeping materials reliable, extending the life of products, and helping with cleaner fuels, deserves attention. Real improvements can come from better handling, smart manufacturing practices, and support for green chemistry. Seeing specialty chemicals like this as part of larger systems, not just isolated ingredients, can spark conversations about safer, longer-lasting, and more sustainable industrial choices.
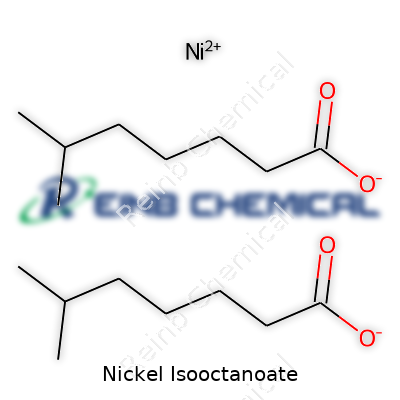
Nickel Isooctanoate has a formula that reads C16H30NiO4. To put that into perspective, the structure features a nickel ion partnered with two isooctanoate groups. Chemists look at it and immediately start considering the carbon and hydrogen content — C16 and H30 — paired with nickel and four oxygen atoms. The link between the nickel and the isooctanoate actually has a big effect on the properties and how it performs in processes like catalysis or additive manufacturing.
Being involved in research and product development has shown me how much false information can easily circulate, especially among students and early-career lab techs. Over the years, it’s become clear that using the right chemical formula isn’t only about passing a quiz or filling out a product sheet. Using the wrong data can put research funding at risk, disrupt industrial production, or even cause equipment failures. Nobody wants to see expensive machinery fail because someone used a cousin compound by mistake.
Nickel Isooctanoate, with its C16H30NiO4 identity, finds its way into lubricants and polymer production. In practical terms, that means understanding the atoms involved is essential for safe handling, environmental compliance, and achieving the performance chemists expect. That alone makes the precise formula data critical, not trivia.
Confusion happens because the word “isooctanoate” sounds a bit like “octanoate,” but they refer to different branched structures. The isooctanoate in this compound matches the 2-ethylhexanoate structure. The nickel couples with two of these groups, forming the salt. Overlooking structural branches can make results from lab tests unpredictable or even dangerous. I’ve seen students lose days to simple transcription errors and companies face delays waiting for proper labeling—all because of chemistry that wasn’t double-checked.
The thing with any metal-organic compound is that the source and purity can shift properties. Safety data and technical sheets rely on that formula. Regulation in most regions pushes for strict documentation precisely because the risks escalate when formulas are misapplied. Exposure levels, reactivity with other additives, and waste management protocols — all hinge on getting the formula right.
Problems pop up in product recalls, environmental issues, and compliance headaches when formulas go unchecked. Companies aiming to export or certify materials, especially in the European Union or North America, need to show exactly what molecules they’re working with. This isn’t only a paperwork exercise; regulators set sterner penalties for mislabeled chemicals with potentially toxic metal ions.
Sometimes, a phone call to the supplier or a chat with a professional chemist makes all the difference. These days, I see more labs running FTIR or NMR checks when anything looks odd. Education and vigilance help avoid expensive errors.
C16H30NiO4, Nickel Isooctanoate, looks uncomplicated. The impact of getting it wrong goes way beyond minor lab mishaps. Accurate formulas let industries innovate without risking safety or running afoul of regulations. Looking up a formula or confirming it with a trusted source doesn’t take time away from progress — it clears the path for better and safer results. That’s something anyone working with chemicals can get behind.
Nickel fits into a strange place in daily life. You see it in coins, batteries, even some food. Nickel isooctanoate, though, usually shows up in industrial settings—think lubricants or catalysts for plastics. Most folks never run into it directly. So, is it hazardous or toxic? That deserves some honest discussion.
Direct contact with nickel isooctanoate rarely happens at home. The bigger risks come in workplaces where people handle or process industrial chemicals. The compound itself doesn’t float around like dust, but if it spills, splashes, or gets on skin or in the air, it can cause trouble.
Nickel irritates skin for a lot of people. Estimates say up to 10% of people across Europe deal with nickel allergy. It leads to redness, itching, sometimes rashes. Nickel compounds, especially soluble ones, reach your body faster than nickel metal alone. Isooctanoate forms can have that risk.
Breathing in nickel over time creates bigger problems. Long-term exposure to certain nickel compounds increases the risk of lung diseases and cancer. Back in the 1990s, researchers pointed to nickel refinery workers having much higher odds of lung and nasal cancers. Occupational Safety and Health Administration (OSHA) and International Agency for Research on Cancer (IARC) both flag nickel compounds as carcinogenic. Data for nickel isooctanoate itself remains limited, but caution around nickel chemicals in general makes good sense.
Nickel doesn’t just disappear when it goes down the drain. If factories fail to control runoff and waste, compounds like nickel isooctanoate wash into soils and waterways. That disrupts fish and aquatic life, and nickel builds up in some plants and animals. Who wants to find heavy metals in their vegetables or local lake fish? Not me.
People working with nickel isooctanoate need more than gloves and goggles. I’ve seen outfits that go cheap on training. They cut corners to save pennies and people end up exposed. Safety procedures belong at the front—even simple things, like using local ventilation or cleaning up carefully, make a real difference. Working with chemicals demands real respect, not just “compliance.”
Labeling matters too. Chemical drums missing warning labels spell disaster. Clear information about risk, first aid, and proper storage keeps people out of trouble. OSHA recommends information sheets easy to read, no fancy language, so folks actually know what they face.
Some newer factories switch to less hazardous compounds where they can. The push for “greener” chemistry sometimes means swapping nickel catalysts with iron or zinc alternatives. Where dropping nickel is impossible, regular checks on air and surfaces keep exposures low. Companies with strong safety culture often see fewer cases of rashes or respiratory problems.
Researchers need to fill in gaps in our knowledge about nickel isooctanoate specifically. Most studies deal with the general class of nickel salts. Honest science and open reporting will help sort out what’s rumor and what’s real risk.
You don’t have to work in a factory to care. Chemicals don’t respect fences, and accidents bleed into communities. Good management today means safer air, water, and health for everyone tomorrow. That’s something worth demanding—no spin, just facts and respect for the risks.
Nickel Isooctanoate doesn’t catch headlines like lithium or cobalt, but across industries, its purity level can mean the difference between a failed process and a trusted final product. Walk into any serious supplier’s product line, and most listings highlight a purity range around 95% to 98%. The remaining fraction tends to hold moisture, trace metals, or sometimes unreacted acids. Anything lower than 95% often faces rejection in lab work or manufacturing, not because chemists are picky, but because results start to wobble.
Think of how a small impurity in a metal can throw a wrench into electronics. The story unfolds the same way with Nickel Isooctanoate. It’s a mainstay in catalysts, coatings, and specialty syntheses, and low-grade batches cause problems that ripple through supply chains. I’ve seen researchers struggle, blaming their methods until they picked apart the reagent grade and spotted the difference. The tiniest contamination in a nickel solution, whether from another transition metal or leftover acid, lingers in finished products and skews results.
Manufacturers aim for high purity not because it’s a badge of honor, but because nickel compounds already walk a regulatory tightrope. Extra metals or solvent residues could mean out-of-spec catalysts or contaminated films. When a process wraps up with fewer surprises, that saves headaches downstream.
Some nickel compounds enjoy strict controls because even tiny contaminants risk affecting process yields or safety. Industry reviews have shown the markets in North America and Europe lean towards third-party purity validation. Source certificates of analysis (COAs) speak volumes. A solid COA lists not just the nickel content, but also the acid value, water content, and traces of copper or iron. I remember having to double-check a new vendor’s batch; their 98% claim looked good, but dig a little deeper, and the iron content lied just above customer specs. Lesson learned: viewing the COA is as crucial as checking the expiry date on milk.
Pure chemistry costs money. Removing every last impurity means expensive distillation and filtration. Demand for ultra-high purity nickel isooctanoate just doesn’t exist outside tight circles, like top-shelf electronics or pharma work, so most industrial and research batches stop near that 98% threshold. Frankly, hitting 99.9% would push production costs higher, and most customers don’t need that level—unless they’re building the sort of gear where any contamination could ruin months of effort.
Suppliers have made big strides by investing in better purification and analytics. Labs make use of modern tools like ICP-OES and GC-MS to root out potential contaminants. Whenever a batch slips up, feedback gets back to the supplier, and sometimes to the producer’s process engineers. These adjustments have steadily increased the baseline quality of commercial stock. Sourcing from established names with good reputations lowers risk, since smaller fly-by-night operations have slipped questionable batches into the market before.
If a process turns fuzzy or batch yields dip, don’t hesitate to ask for batch-specific COAs and clarification on storage conditions. Request a purity breakdown that covers major and trace impurities. For research teams, running a quick check with available in-house techniques (like IR or even simple gravimetric nickel determination) can place full confidence in a new supplier. Open communication and strong documentation continue to close the gaps between vendor promises and lab results, which is crucial as markets expect tighter tolerances every year.
Working alongside specialty chemicals brings a sense of responsibility. Nickel isooctanoate sits in that category, thanks to its unique properties and the very real hazards attached to improper handling. This compound finds its way into catalysts and coatings but comes with health and environmental concerns that can’t be brushed aside. Being deliberate with how it’s stored and handled isn’t about bureaucratic boxes to tick; it’s about keeping lives and the planet out of harm’s way.
Many folks who store chemicals come to appreciate the value of a designated chemical storage cabinet. I’ve seen too many small shops where solvents and metal solutions share makeshift shelves. With nickel isooctanoate, sloppy storage turns into a risk for fire, inhalation, or leaks. Keep containers tightly sealed in well-ventilated and cool rooms, away from combustible or incompatible materials, especially acids and oxidizing agents.
Labeling also deserves respect. Labels fade or peel, and then confusion sets in. Anyone pulling a canister from the shelf should see exactly what’s inside. I always recommend waterproof marker labels and clear, legible hazard symbols. Record-keeping—dates in and out, batch numbers—identifies who’s responsible if something goes awry.
The first time I helped move metal-organic compounds, I underestimated fumes. Those coughing fits teach a quick lesson. A full set of PPE (protective gloves, chemical-resistant apron, goggles, and a fitted respirator) blocks contact and helps workers keep their health. Rushed handling often leads to splashes or drips, and the fewer chances skin and lungs have to meet nickel compounds, the better.
Handwashing stations close by aren’t a theoretical nice-to-have. The fastest way to stop nickel from traveling onto food, doorknobs, or your face is simple soap and water, right after removing gloves. Clean clothes, too, so contaminated uniforms don’t tag along home.
Fume hoods and good ventilation matter the same way that strong locks protect valuables. Nickel isooctanoate can vaporize or “off-gas” under warm conditions, contaminating room air. Facilities that handle it regularly should invest in exhaust systems—just cracking a window won’t cut it. This kind of protection keeps both workers and those outside the immediate space out of the exposure zone.
Spills feel inevitable in a bustling workspace. Spill kits for organometallics aren’t optional here. Absorbent pads, proper neutralizers, and step-by-step cleanup checklists prevent a small accident from turning into a workplace incident. Routine drills help, too. Staff should know how to contain and report any leak without panic or delay.
Nickel lingers. Pouring leftover chemicals down the drain isn’t just illegal in most places; it pollutes water for everyone. Waste contractors who specialize in hazardous substances know exactly how to neutralize, transport, and dispose of such materials. It may cost the business a bit more up front, but paying for a cleanup or hospital bill costs far more in the long run.
Some companies encourage recycling of empty containers. In my experience, it’s safer to treat every can or drum as contaminated. Rinse them in designated waste baths, label as hazardous, and don’t re-use for other materials.
I’ve seen the difference when a workplace makes chemical safety a habit rather than an afterthought. Regular training keeps risks at the front of people’s minds. Open conversations about safety concerns let workers speak up, suggest improvements, and spot problems early. It’s not just about following the rules—it’s about looking out for each other and respecting the invisible dangers we work with every day.
| Names | |
| Preferred IUPAC name | Nickel 2-ethylhexanoate |
| Other names |
Nickel(II) isooctanoate Nickel 2-ethylhexanoate Nickel octoate Nickel(II) 2-ethylhexanoate |
| Pronunciation | /ˈnɪkəl aɪˌsəʊˈɒktəˌneɪt/ |
| Identifiers | |
| CAS Number | 938-92-1 |
| Beilstein Reference | 1908734 |
| ChEBI | CHEBI:131183 |
| ChEMBL | CHEMBL4294660 |
| ChemSpider | 61454 |
| DrugBank | DB11268 |
| ECHA InfoCard | ECHA InfoCard: 100.254.257 |
| EC Number | 276-380-4 |
| Gmelin Reference | 67692 |
| KEGG | CHEBI:131537 |
| MeSH | D009563 |
| PubChem CID | 153308395 |
| RTECS number | QU4375000 |
| UNII | 2II3O66F6G |
| UN number | UN3082 |
| CompTox Dashboard (EPA) | UVC00018472 |
| Properties | |
| Chemical formula | C16H30NiO4 |
| Molar mass | 470.235 g/mol |
| Appearance | Green liquid |
| Odor | Characteristic |
| Density | 0.927 g/mL at 25 °C |
| Solubility in water | Insoluble |
| log P | 3.7 |
| Vapor pressure | <0.1 mm Hg (20 °C) |
| Basicity (pKb) | 6.38 |
| Magnetic susceptibility (χ) | +2200e-6 cm³/mol |
| Refractive index (nD) | 1.464 |
| Viscosity | Viscosity: 15-20 cSt (at 40°C) |
| Dipole moment | 1.87 D |
| Pharmacology | |
| ATC code | V07AB44 |
| Hazards | |
| GHS labelling | GHS02, GHS07, GHS08 |
| Pictograms | GHS07, GHS08, GHS09 |
| Signal word | Danger |
| Hazard statements | H317: May cause an allergic skin reaction. H351: Suspected of causing cancer. H411: Toxic to aquatic life with long lasting effects. |
| Precautionary statements | P261, P264, P272, P280, P302+P352, P333+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1 2 0 |
| Flash point | 103 °C |
| Lethal dose or concentration | Lethal dose or concentration: LD50 (oral, rat): > 5,000 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Nickel Isooctanoate: Oral rat LD50 > 5000 mg/kg |
| NIOSH | NIOSH: QR3500000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Nickel Isooctanoate: 1 mg/m3 |
| REL (Recommended) | 200 mg Ni/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Nickel(II) 2-ethylhexanoate Nickel octanoate Nickel neodecanoate Nickel naphthenate Nickel acetate Nickel(II) stearate |