
Potassium isooctanoate came onto the industrial radar as a response to the push for specialty surfactants that could manage both sustainability and performance in demanding applications. Decades ago, formulators turned to potassium salts of fatty acids for their biodegradability and safety compared to heavier, more hazardous metal salts. The journey toward isooctanoic acid derivatives marks a chapter where the chemical industry explored branching chain carboxylates as alternatives to linear ones, aiming for unique solvency and wetting profiles. Over time, researchers fine-tuned production, preferred isooctanoic acid over simpler fatty acids, and realized the value of the potassium salt for water solubility and gentle reactivity. Chemistry labs, from small university setups to the largest multinational corporations, learned the nuances of controlling purity, color, and trace byproduct levels, pushing the product closer to market standards and regulatory compliance.
What sets potassium isooctanoate apart is its molecular architecture. Built from an eight-carbon, branched-chain carboxylic acid and neutralized with potassium hydroxide, it carries both hydrophilic and hydrophobic traits. This duality helps the compound act as a versatile surfactant—finding its stride in cleaners, paints, lubricants, agrochemical preparations, and even as an intermediate in the synthesis of esters. The compound’s chain branching leads to lowered melting points and unique phase behaviors, standing out in formulations where temperature-sensitive stability matters. Every batch targets strict assays—not just for purity but for color, stability, and ease of handling.
Potassium isooctanoate usually appears as a white to off-white powder or can sometimes take a pasty consistency, especially under high humidity. Its scent carries a faint, slightly oily note, a legacy of the precursor acid. The potassium salt form dissolves readily in water, giving clear to slightly hazy solutions, depending on load and impurity content. This solution swings alkaline, a trait exploited for saponification and cleaning ability. Thermal stability holds up well under typical processing conditions, tolerating moderate heat without significant decomposition. Its branched structure brings solid-state mobility and contributes to lower hygroscopicity than straight-chained analogs, making storage less of a headache.
Certification bodies and regulatory frameworks demand a transparent technical dossier for potassium isooctanoate. Purity commonly runs above 98%, with potassium content, residual isooctanoic acid, moisture, and color measured in each lot. Labels communicate origin, batch number, date of manufacture, and shelf life, while safety data sheets describe hazards, first aid, and handling guidance. Product packaging describes corrosion sensitivity, and shipments must protect the material from excessive humidity and exposure to incompatible chemicals. Channel partners rely on this information for regulatory filings, customs clearance, and process safety analysis.
The most reliable commercial process starts with isooctanoic acid, sourced via branched alkyl oxidation or hydroformylation and subsequent oxidation, followed by neutralization with high-purity potassium hydroxide. Synthesis calls for controlled temperatures, slow addition, and efficient mixing to avoid local excesses that could trigger color changes or unwanted byproducts. Water gets added or removed to reach the desired concentration, and filtration or centrifugation takes out insoluble material. In the lab, each step gets tracked by titration, chromatography, and gravimetric analysis. Waste management includes neutralizing excess alkaline material and capturing volatile residues for proper treatment.
Chemists tweak potassium isooctanoate for wider utility, exploiting its surfactant properties or adapting it as an intermediate. Esterification produces isooctanoic esters, valued for their lubricity and volatility control. Under oxidizing conditions, it breaks down into smaller acids or alcohols, granting a route to specialty molecules. Sometimes, crosslinking or co-salting with other metal ions adjusts solubility or activity, feeding innovation in coatings and plasticizers. Each modification pathway gets mapped, risks assessed, and downstream impact modeled before scale-up or commercial release.
Potassium isooctanoate goes by multiple synonyms depending on the market or application—potassium 2-ethylhexanoate, potassium caprylate, and potassium octanoate among the most common. Certain suppliers coin proprietary names for blend products or enhanced-purity grades, sometimes referencing “EHA” for the 2-ethylhexanoic backbone. Regulatory filings and niche catalogs may use IUPAC nomenclature or regional branding, so procurement pros often cross-reference against CAS numbers and functional requirements.
Any operator handling potassium isooctanoate must respect basic chemical hygiene. Skin exposure rarely marks a danger, but eye and mucous membrane contact could irritate, thanks to its basicity. Labs and plant workers wear gloves and goggles, storing the compound in sealed containers away from acids and oxidizers. Local regulations often require ventilation for dust control; some larger facilities monitor workplace airborne levels to ensure compliance. Emergency spill kits feature absorbents and neutralizing agents. Disposal lines up with other organic salts, usually after neutralization and effluent testing. Training programs focus on symptom recognition, first aid, and quick containment to prevent escalation.
This salt powers a host of everyday and industrial products. Paint manufacturers prize its role as a drier and dispersant, helping pigments develop vivid color and smooth out flow characteristics. In coolants and hydraulic fluids, it helps buffer pH and stop corrosion, lengthening equipment life and reducing downtime. Cleaning product formulators appreciate its balance of water solubility and oil-breaking force, making it useful in degreasers, detergents, and even fire-fighting foams. Food and pharma remain off-limits due to limited toxicology data, but agricultural chemists see value in using it to stabilize pesticide and fungicide slurries, controlling release and preventing aggregation. My own hands-on time in coatings applications highlighted its ability to control froth and wetting, sometimes outperforming more expensive specialty surfactants in grinding and let-down stages.
Innovation rarely stops, and over the years, potassium isooctanoate sparked curiosity for its potential in green chemistry and advanced material science. Researchers view branched potassium carboxylates as test beds for biodegradable surfactants, finding ways to tailor hydrophile-lipophile balance for custom applications. Development teams push boundaries in polymerization, electroplating, and nano-dispersion, drafting formulations that utilize the unique phase characteristics and reactivity of the salt. Sustainability metrics—carbon footprint, life cycle assessment, water use—now join old-school efficiency on the priority list. Academic-industry partnerships try to crack new uses, from advanced lubricants to high-performance adhesives, pivoting toward a future where more benign chemistries displace traditional toxins.
Toxicologists take a cautious approach with potassium isooctanoate. Acute oral and dermal exposures in standard animal models show low to moderate toxicity, contingent on dosage and formulation. Chronic exposure remains under-studied, especially when it comes to metabolites generated by microbial or environmental action. No clear evidence yet flags this salt as a major environmental or human health risk, but the growing demand for safer workplace environments and transparent ingredient lists means ongoing investment in deeper toxicology and eco-toxicology studies. Ingredient approval for new applications often hinges on data from these programs. In my view, the community would benefit from more third-party studies and broader access to real-world exposure data for validation.
The horizon holds promise and some challenge. As chemical manufacturers deal with pressures to clean up supply chains and cut hazardous waste, potassium isooctanoate’s profile delivers a head start—biodegradable backbone, moderate reactivity, and compatibility with green chemistry principles. Still, new synthetic catalysts, recycling processes, and renewable feedstocks could shrink emissions and boost efficiency even further. Product line extensions, like high-purity or multi-metal blends, seem likely, targeting even tighter regulatory demands and niche performance targets. Meanwhile, the push for greener surfactants in construction, transportation, and consumer goods prompts investment in application engineering, safety validation, and regulatory acceptance. Industry leaders with an eye on product stewardship and cross-disciplinary R&D will shape where potassium isooctanoate lands in the next decade.
Potassium isooctanoate might sound like something tucked away in a chemist’s backroom, but its reach shows up in daily life more than most folks realize. People working in agriculture, cleaning, and industry bump into this compound without giving it much thought. I first heard about it from a friend who manages municipal water treatment—he swears by its role in helping systems run cleaner and safer. After digging deeper, it’s clear this little molecule pulls its weight.
Most industrial cleaners count on surfactants, and potassium isooctanoate often gets the nod. Surfactants let water and grease mix, making grime easier to wash away. The unique balance in this molecule helps break through tough oil or dirt. Everyday folks might not see the direct impact unless they work in food processing or heavy machinery. Still, food safety and machinery uptime both rely on strong, stable cleaners, especially ones that do their job at a wide range of temperatures.
Farms face constant pressure from pests and plant diseases, and many growers turn to potassium isooctanoate as a safer alternative to older, more damaging crop protection agents. It doesn't hang around in the soil like some synthetic options from earlier decades. Regulatory agencies, including the EPA, have noted its lower toxicity compared to harsher chemicals. I met a vineyard worker in California who said his supervisor picked products with potassium isooctanoate specifically to stick with organic certifications. This detail alone can mean the difference between a crop hitting premium markets or just scraping by in bulk bins.
Water treatment plants need solutions that break down contaminants but also remain safe for aquatic life. Potassium isooctanoate comes up in sludge dewatering and helps keep everything flowing smoothly. Utilities often share data on the need for agents that don’t add more pollutants. The environmental impact gets measured down to trace levels, and research shows that this compound doesn't persist like older, riskier additives. That keeps rivers and lakes steadier for fishing, recreation, and drinking water.
Not all is perfect. High concentrations of potassium isooctanoate can still cause skin and eye irritation, so workers always need solid training and respect for safety gear. In my experience working in warehouse supply, manufacturers cycle through periods of tight inventory, especially when chemical feedstocks get pinched by global disruptions. This stresses the ongoing need for reliable sourcing and clear labeling, so nobody gets shorted during busy season or ends up with counterfeit supply.
Demand for safe, effective surfactants isn’t going away. More companies invest in products that meet both safety and environmental regulations. Researchers keep an eye on potential side effects if concentrations drift beyond recommended levels. The up-and-coming generation of chemists pushes for even more biodegradable options derived from renewable raw materials. Open research and informed public discussion about chemicals like potassium isooctanoate let consumers and professionals make safer, smarter choices. There’s always room for improving oversight so this compound continues to earn its place in critical applications without unwanted surprises.
Potassium isooctanoate pops up in cosmetic ingredient lists, household cleaners, and some industrial settings. It serves as a surfactant, helping mix oils, dirt, and water. This sounds technical, but it just means it allows products to work better on our skin or surfaces. The question often arises: is it safe to have this chemical against our skin? Most people barely think twice about it, especially since it isn’t a household name like sodium lauryl sulfate or glycerin. That makes digging into available safety information even more important.
In reading through the database at the Cosmetic Ingredient Review (CIR), potassium isooctanoate hasn’t raised red flags. Limited human patch testing reports few to no cases of irritation at concentrations used in personal care products. It breaks down into potassium ions and isooctanoic acid on the skin. Potassium appears all over the body in much higher amounts, and isooctanoic acid belongs to a family of compounds found in animal fats and some food additives. This lowers the worry that small exposures from consumer products would lead to harm.
I asked a dermatologist friend about this substance. She shrugged and said she doesn’t remember seeing reactions in her patients, even those with allergies or eczema. I don’t take one person’s experience as universal proof, but no giant spike of complaints lines up with the available literature.
Reports from industrial settings give a slightly different view. If someone handles concentrated potassium isooctanoate for hours without gloves or proper ventilation, it can cause irritation. At home, people do not usually encounter those raw concentrated forms. Still, labels on cleaning products often recommend wearing gloves not only for this ingredient but for many surfactants and solvents in the bottle. I’ve had dry, peeling hands after cleaning marathons even using mainstream cleaners, mostly from the stripping effect on my skin’s oils, not a specific allergic reaction.
The U.S. Food and Drug Administration doesn’t restrict the use of potassium isooctanoate in personal care products when used in amounts typically found on shelves. The European Chemicals Agency also lists it as a low-risk substance regarding contact with skin. By law, finished products must go through formula safety assessments before landing in stores. Consumer complaints or injury reports get tracked by poison control centers and regulatory bodies, which helps catch patterns early.
For folks with sensitive skin or a history of allergies, patch testing new products still makes sense. Trying a dab on the inside of the arm gives a pretty good idea of what to expect on the rest of the body. If redness or stinging shows up, it’s best to stop using it. For healthy skin, the vast majority of people will not run into problems. Anyone regularly exposed to larger amounts through work should wear gloves and wash up carefully. Dryness doesn’t always mean a product is unsafe, but keeping skin moisturized protects against long-term irritation.
Years of monitoring have not revealed major problems from potassium isooctanoate in consumer products. The best approach stays the same as with most modern chemicals: read the label, use as directed, and listen to your body’s signals. If industry or science uncovers new evidence, regulatory agencies review and update recommendations. Until then, for most people, potassium isooctanoate remains a low concern in daily life.
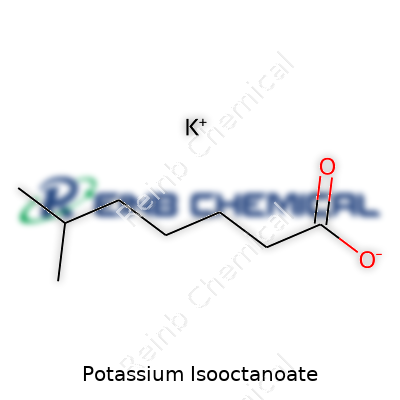
Potassium isooctanoate comes together when you react potassium hydroxide with isooctanoic acid, better known among chemists as 2-ethylhexanoic acid. This kind of reaction swaps out a hydrogen atom for a potassium atom, forming a salt. The resulting chemical wears the formula C8H15KO2. That means its molecule holds eight carbons, fifteen hydrogens, one potassium, and two oxygens. In a jar on the shelf, it would show up as a white to off-white powder or possibly a solid chunk, depending on the conditions.
Every line and curve in a chemical formula matters. The backbone of isooctanoate, with its branching structure, resists breaking down in water and interacts differently with other compounds than a straight-chain fatty acid would. People who work with chemical processes often care about these differences because the formula shapes how potassium isooctanoate goes about its business: whether it helps keep a mix stable, changes a reaction speed, or does something as simple as preventing foam in an industrial vat.
Manufacturers have counted on potassium isooctanoate as an emulsifier, a corrosion inhibitor, and as an anti-foaming agent in industrial settings for years. Its unique makeup keeps it safe for use in certain applications, while also posing tricky questions for environmental safety. From my own time working with specialty chemicals, I know how easy it is to choose the wrong salt when the subtleties aren’t clear. There’s a reason many professionals double-check not just the name on the order sheet, but also the formula behind it.
Potassium isooctanoate’s role in preventing engine parts from rusting, for example, saves countless dollars in repairs. In another corner, paint producers use it as a stabilizer, turning out finely mixed, long-lasting coatings. Yet even those successes raise their own hurdles. Questions keep popping up about the safe handling of the chemical and its breakdown products. Some researchers have raised flags about possible byproducts, especially when these substances get into streams or groundwater. Communities living near factories sometimes press for more data on how these compounds move through soil and into food or water sources.
Solving these challenges means drawing on the lessons of both chemists and everyday workers. Regular monitoring—checking water near plants, testing soils—lets companies catch issues before they build up. Training remains essential. Not every mix-up sits in the chemistry; sometimes it’s a labeling mistake, a spill missed during cleanup, or a storage issue. Training shifts a culture toward safety, turning long-winded lectures into careful habits when handling even familiar salts.
Researchers ought to keep exploring less persistent alternatives to potassium isooctanoate, especially in uses that could lead to broad environmental exposure. Switching to greener options, when possible, doesn't just cut risk; it helps rebuild trust with communities. Investment in safer processes or closed-loop recycling systems can also shrink a company’s environmental footprint. From my experience, the best solutions come when both safety and practicality have a seat at the table.
Potassium isooctanoate pops up in plenty of cleaning products and industrial settings. People hear about new chemicals and start to wonder—What happens after we wash them down the drain? Do they stick around in nature, or do they break down and disappear? Concerns about what really counts as “biodegradable” crop up not only among scientists, but also regular folks who care about water, soil, and the air we breathe.
Kick off with what this stuff does. It’s basically a salt formed from isooctanoic acid and potassium. Companies use it for its abilities as a cleaning agent and surfactant—it helps oils and water mix, helping to lift grime and dirt. Non-chemists run into it in car care, floor cleaners, and sometimes even as an emulsifier for paints.
Lots of manufacturers slap “biodegradable” on their packaging, but that term can get slippery. True biodegradability means microorganisms, like bacteria and fungi, munch the chemical and turn it into basic stuff such as carbon dioxide, water, and harmless minerals. Think about compost piles—the banana peel disappears, but a plastic fork sticks around. Potassium isooctanoate, though, behaves more like the peel.
Lab tests and some industrial studies show this compound breaks down pretty fast under the right conditions. Researchers have tested potassium isooctanoate in both aerobic (oxygen-rich) and anaerobic (low-oxygen) settings, and the results often show impressive microbial action. Within a few days or weeks, most of the chemical vanishes from test samples, leaving only simple end products. Biodegradation rates can change with temperature, pH, and the unique microbe “community” in the soil or water, but the trend holds up: It doesn’t stick around for long.
Having a cleaner that’s biodegradable doesn’t mean it’s always safe to dump buckets into the yard. Breaking down quickly is just the first test. It matters what happens next. The smaller byproducts (like short-chain acids or salts) also need to be harmless. Otherwise, we’d swap one problem for another. Research has found potassium isooctanoate’s breakdown products don’t show toxic effects in realistic concentrations. That’s probably why EU chemicals safety agencies have given it a thumbs-up, and why the US Environmental Protection Agency lists it as “low concern.”
Just because potassium isooctanoate breaks down doesn’t mean we get a blank check. Overspray and misuse can still hurt plants or aquatic life by overloading local waterways. Industry can pitch in by using the smallest effective amount. Local water treatment plants already do their part—microbes in those systems help finish the job.
For consumers, reading labels and not using more than needed means less extra potassium, isooctanoate, or any other chemical making its way into storm drains. Environmental groups and regulatory agencies keep an eye on these substances with updated research, nudging companies toward ever-safer formulas.
Plenty of us care about the products we use at home or work. Potassium isooctanoate’s ability to break down is good news, but the goal keeps moving—safer chemicals, less pollution, and solutions that line up with modern values. Staying informed, using products responsibly, and encouraging innovation matter for both cleaner homes and a cleaner planet.
Potassium isooctanoate crops up in a handful of specialized industries as a surfactant and chemical intermediate. Chemically, it’s a potassium salt of a branched fatty acid, mostly appreciated for how it breaks down oils in industrial cleaning and sometimes in specialty chemical applications. Its use pops up in metalworking, textile processing, and more rarely, in household products. Those experiences do not directly translate to food or medicine, and for very good reasons.
Plenty of folks care about what goes in their bodies. Food additives run a tough gauntlet of approval worldwide. The FDA and similar agencies in Europe pore over toxicity, metabolism, and allergy potential. Any salt or acid that goes near a food label must pass both safety and practical food technology hurdles. In my previous work in food tech, I saw lengthy ingredient dossiers; compounds needed low toxicity, clean taste, no off-odors, and proven records of long-term safety. Potassium isooctanoate doesn’t appear on the extensive lists of permitted food additives in the US, EU, or Japan. Nobody’s using it as a preservative, emulsifier, or stabilizer in mainstream food production.
Most reasons point toward its chemical structure and history. The fatty acid backbone—a short, branched chain—can introduce untested metabolic byproducts. Metabolism in the human body often handles straight-chain fatty acids just fine, but branches might break down differently, possibly leading to unusual or unsafe metabolites. There’s no public data showing it won’t build up or cause harm after years of low-level exposure, which rules it out for serious consideration as a food additive. Consumers want proven, time-tested ingredients; regulators play it safe with untested chemicals.
Medicine brings even stricter standards. Each ingredient in a pill or syrup undergoes toxicology studies, carcinogenicity testing, and impurity monitoring. Potassium salts sometimes help buffer solutions or stabilize formulations. Not all potassium compounds make the cut: ones with long-standing safety records (potassium chloride, potassium citrate) show up time and again, while unproven compounds get shut out early if they introduce risk.
Potassium isooctanoate falls in a gray area. No evidence emerges from regulatory databases or reputable drug compendia. No approved products list it as an excipient. Absent a strong safety profile and direct regulatory sign-off, the chances of finding it in a pharma lab or finished drug remain slim.
Anyone pitching a “new” ingredient for food or drugs must show more than chemical similarity to approved substances—they need comprehensive data on what the compound does in the human body. Without that assurance, companies and regulators won’t touch it.
Diving into safety studies would clear up the confusion. Animal and cell culture testing would need to come before considering this salt for any edible or medicinal use. Long-term toxicity and metabolism experiments take time and money, and unless there’s a compelling reason—better performance, cheaper cost, or clear consumer benefit—companies usually stick with established compounds. At home, I stick to familiar names on my medicine and food labels.
So, potassium isooctanoate hasn’t made its case for food or pharma. Experience says stick with ingredients offering proven safety. If future research turns up positive evidence, then regulators might reconsider. Until then, it belongs in factories, not kitchens or pharmacies.
| Names | |
| Preferred IUPAC name | Potassium 3,5,5-trimethylhexanoate |
| Other names |
Potassium 3,5,5-trimethylhexanoate Isooctanoic acid, potassium salt |
| Pronunciation | /pəˌtæsiəm aɪˌsoʊˈɒktəˌnoʊeɪt/ |
| Identifiers | |
| CAS Number | ["25168-26-7"] |
| Beilstein Reference | 3583666 |
| ChEBI | CHEBI:85721 |
| ChEMBL | CHEMBL3638622 |
| ChemSpider | 10978198 |
| DrugBank | DB14672 |
| ECHA InfoCard | 100.237.485 |
| EC Number | EC 253-066-3 |
| Gmelin Reference | 51039 |
| KEGG | C14325 |
| MeSH | D018150 |
| PubChem CID | 23672584 |
| RTECS number | WH7000000 |
| UNII | M0K1146ECW |
| UN number | UN3265 |
| CompTox Dashboard (EPA) | DTXSID9020362 |
| Properties | |
| Chemical formula | C8H15KO2 |
| Molar mass | 186.31 g/mol |
| Appearance | Clear colorless to yellowish liquid |
| Odor | Odorless |
| Density | 0.99 g/cm3 |
| Solubility in water | Soluble |
| log P | 0.3 |
| Vapor pressure | Negligible |
| Acidity (pKa) | pKa ≈ 4.9 |
| Basicity (pKb) | 2.56 |
| Refractive index (nD) | 1.421 |
| Viscosity | 2.6 mPa·s (20 °C) |
| Dipole moment | 2.94 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 417.46 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -658.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | −6257.7 kJ/mol |
| Pharmacology | |
| ATC code | A16AK10 |
| Hazards | |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Flash point | > 100 °C |
| Lethal dose or concentration | LD50 (oral, rat): > 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral >2000 mg/kg |
| NIOSH | No NIOSH. |
| PEL (Permissible) | PEL: Not Established |
| REL (Recommended) | 0.1 – 0.6% |
| Related compounds | |
| Related compounds |
Potassium caprylate Potassium 2-ethylhexanoate Potassium octanoate Sodium isooctanoate Isopropyl isooctanoate |