
Years back, the chemical industry worked with only a handful of surfactants, many derived from natural fats or petroleum-based chains. Sodium isooctanoate entered the scene as companies searched for specialized alternatives with improved solubility, better compatibility, and minimal residue. The compound found attention due to its branched carbon chain, which pointed toward stability and unique surface activity. Stories from early technical bulletins in the 1950s and 60s show researchers tinkering with carboxylates, finding that branching with isooctanoic acid led to compounds that could tolerate wider temperatures and harder water. Fast-forward to the present, and sodium isooctanoate stands on shelves as a tailored choice for businesses needing something more than commodity surfactants.
Sodium isooctanoate is a fine example of a specialty surfactant. This white, sometimes slightly off-white powder dissolves easily in water, forming solutions that change the behavior of other chemicals at surfaces and interfaces. Its molecular backbone carries both hydrophilic and hydrophobic groups, making it useful in places where ordinary soaps and detergents fall short. Different grades exist, each geared for either industrial or research use, yet all share the core functional feature: reliable wetting and emulsifying power. Producers market sodium isooctanoate under a range of trade names, owing to its adaptability and proven performance where ordinary agents lag.
A chunk of sodium isooctanoate’s appeal traces back to its physical characteristics. Its melting point stays comfortably above room temperature, and it doesn’t clump excessively under common storage conditions. Chemically, the sodium salt end brings high solubility, even in moderately hard water, letting it mix in fast. The octanoate backbone, with its eight carbon atoms and branching, adds stability against breakdown and a mild but distinctive fatty aroma. pH in solution lands in the neutral to mildly basic range, which sits well with most commercial formulas. The branched structure resists foam collapse, which matters in cleaning and lubrication.
True compliance calls for clear labeling. Labels list sodium content, purity, residual solvents, and any trace by-products. Manufacturers guarantee minimum assay values, often above 98%, and mark the presence of water as either moisture content or loss on drying. Safety data appears on both packaging and datasheets, often in line with international standards like GHS or REACH. Proper batch identification lets buyers trace product origin, giving peace of mind in regulated industries. Regulatory frameworks demand hazard pictograms, signal words, and precautionary statements—nothing less keeps supply chains transparent and legal.
Most modern processes start with isooctanoic acid, which reacts with sodium hydroxide or carbonate in water. The reaction yields sodium isooctanoate and water, with careful control over pH to avoid excess base. Scaling up for industry takes temperature control and special mixing, since uneven heat or too rapid addition can clump the product or reduce yield. The end material is filtered or centrifuged, then dried under reduced pressure to deliver the free-flowing powder that customers expect. Waste streams, mostly water with trace organics, run through controlled neutralization before disposal or reuse.
Chemists like to push molecules for more. Sodium isooctanoate reacts with acidic or electrophilic compounds, forming esters or mixed salts on demand. Those in polymer science hit it with acrylates to add surface wetting to plastics. Others cap the carboxyl end to tweak solubility or resistance to chemicals. In laboratories, this compound stands as a model carboxylate anion, helping undergraduates visualize ion pairing and micelle formation. It doesn’t oxidize quickly, so shelf life stays long, and branching fends off degradation that plagues straight-chain alternatives.
Browsing supply catalogs, sodium isooctanoate comes under several listings. Some call it the sodium salt of 2-ethylhexanoic acid, while others shorten things to ‘sodium caprylate (branched)’. Certain brands tie in trade names, capitalizing on its fatty acid heritage, but they all refer to the same core structure. The variety in naming often reflects different markets—biotech, coatings, or cleaning—all eyeing the compound for its standout features.
No one takes shortcuts with chemical safety. Sodium isooctanoate, like similar surfactants, can irritate the skin or eyes at high concentrations. Factories store it in sealed drums, away from strong acids or oxidizers. Workers need nitrile gloves, goggles, and dust masks during handling. Safety protocols mandate showers and eyewash stations, since splashes aren’t rare during mixing or transfer. Spill kits and absorbents sit nearby. International shipping calls for documents certifying the material as non-hazardous for transport under most regulations, but transporters train for emergencies all the same.
Industry relies on sodium isooctanoate in more products than most people realize. In metalworking fluids, it stabilizes emulsions while limiting corrosion. Textile factories use it to mix dyes more evenly and soften finish baths. Lubricant blenders reach for it when they need additives that won’t gum up engines. Some detergents, designed for machines under harsh conditions, only clean well with this compound standing guard. Laboratories keep sodium isooctanoate as a control surfactant, comparing new molecules for detergency, biodegradability, or toxicity. Its low odor and stable foam help it elbow out more volatile or less effective surfactants in dozens of recipes.
Universities and companies keep studying sodium isooctanoate, testing new uses or seeking out modified versions. Assisted by advanced techniques like NMR and GC-MS, researchers map out how this molecule adsorbs onto metals or reacts on enzyme surfaces. In pilot plants, teams try to stretch its role in green solvent systems or as a dispersant for nanoparticles. Some projects shift the synthetic route to renewables, replacing petro-derived feedstocks with bio-based acids. The aim stays the same—better environmental performance, cost savings, or improved technical results. Conferences and journals continue to carry new reports, often tied to patent filings.
Few chemicals escape scrutiny over safety. Toxicity studies for sodium isooctanoate show that, at customary dosages, the risk to humans and animals stays low. Acute oral or dermal toxicity falls into the ‘mild’ range, with irritation the main concern for concentrated forms. Fish and aquatic invertebrate studies send up caution signals only at much higher levels than typical product use. Regulatory agencies track environmental breakdown, steering the industry to keep releases to a minimum. Ongoing assessments update safety profiles, giving buyers and regulators up-to-date benchmarks.
Looking ahead, sodium isooctanoate stands to play a role in sustainable chemistry. As demand rises for bio-based surfactants and reduced-toxicity additives, producers work to reduce impurities and carbon footprints. Emerging applications in waterborne coatings, advanced lubricants, and controlled-drainage irrigation point to a broader reach. Improved analytical tools let developers custom-tailor the molecule for new industries, including electronics and biomedicine. Market data shows a steady climb in demand, nudged by stricter regulations on older detergents and greening commitments from global brands. The challenge stays in scaling up production without spiking costs or raising safety risks. As teams close the gap between lab breakthroughs and factory floors, sodium isooctanoate pushes forward, answering practical needs rooted in decades of know-how.
I used to rush through the ingredient lists on bottles around the house, assuming every chemical might be a risk or some mysterious filler. Sodium isooctanoate caught my eye a few years back while looking for gentler alternatives for my skin. Turns out, this unremarkable name comes with a few interesting stories about what goes on in products we rely on every day.
This compound does more than just sit in the background. You’ll often find it in household cleaners, shampoos, shower gels, and even dishwashing liquids. It acts as a surfactant—helping water mix with oils and dirt, creating a foam that lifts away grime. That sounds simple, but it makes a big difference for anyone dealing with hard water stains in the bathroom or kitchens where grease builds up fast.
People get nervous about chemical names. Maybe they imagine harsh reactions or long-term health concerns. I’ve pulled ingredient safety data before buying, and sodium isooctanoate doesn’t fuel the usual red flags. Research led by regulatory agencies finds it safe for the uses approved. Its molecular shape makes it less likely to irritate skin than harsher surfactants, which honestly matters to me as someone prone to dry hands. Dermatologists often recommend milder formulations for families with sensitive skin, and gentle surfactants like this one check that box.
Manufacturers need ingredients that work across a range of products. I’ve seen formulators opt for sodium isooctanoate because it helps reduce residue, washes out cleanly, and keeps detergents stable during storage. The big win for the consumer is fewer streaks on dishes, better foam in soaps, and fewer mystery rashes from using cheaper alternatives. It’s also biodegradable, breaking down into substances that don’t linger in the environment. For households with septic systems or anyone worried about chemical runoff, this is a real advantage.
With more people asking how their buying choices affect rivers and soil, sodium isooctanoate stands out for breaking down more quickly than older synthetic surfactants. Still, any chemical can cause issues when dumped in massive amounts. We all have a role to play: using only what’s needed and supporting brands that publish real safety data. Clear labeling helps, letting families avoid compounds they know don’t suit them. Some companies have started offering full ingredient explanations online—something I look for now before picking up a new cleaner.
As consumers gain more control over what ends up on their shelves and skin, sodium isooctanoate shows that not every complex-sounding ingredient hides a danger. With open information and careful sourcing, shoppers can make smarter choices and keep harmful chemicals out of homes and waterways. Companies that back up their claims with peer-reviewed studies and transparent data make it easier for all of us to trust what goes into our cleaning routines.
Skincare shelves display a parade of chemical names. Sodium isooctanoate probably doesn’t ring any bells for most shoppers, but it’s tucked into some cleansers, shampoos, and specialty treatments. This ingredient steps in for two reasons: it helps dissolve grime and keeps formulas stable. Its origins? A synthetic salt based on isooctanoic acid. Many ingredients based on fatty acids share a strong track record, but smart consumers pay attention to each one.
Dermatologists usually point to sodium isooctanoate as a mild surfactant—meaning it helps water and oil mix to rinse debris off your skin. Think of surfactants as helpful kitchen sponges; some strip everything, others treat a surface gently. Sodium isooctanoate lands on the gentler side. Safety regulators and independent groups, including the Cosmetic Ingredient Review panel, have reviewed its chemical family without red flags. They rely on peer-reviewed studies and decades of product data for these calls.
I’ve noticed that people with eczema or super-sensitive skin often stay skeptical of every ingredient—rightfully so. There aren’t many headlines about sodium isooctanoate causing massive allergic reactions or breakouts, even for sensitive folks. Still, everyone’s skin is unique. A patch test on a small area tells you more about your personal response than any research paper.
Cleansers and some moisturizers use sodium isooctanoate for its blend of cleaning power and skin compatibility. Unlike classic sulfates (the famous bubbly cleansers that sometimes trigger dryness), sodium isooctanoate tends to be less irritating. I’ve worked with people who grew frustrated after trying “gentle” cleansers, only to find hidden sulfates or heavily fragranced chemicals. Switching to products using milder surfactants, including sodium isooctanoate, often brought relief and kept skin from feeling stripped.
Reviewing ingredient lists pays off. Many “clean” or hypoallergenic products take advantage of this ingredient’s stability and cleaning performance. Checking labels helps people avoid harsh formulas and spot anything new or unfamiliar. If you contact a brand for more details or request sample sizes, you learn more about your skin’s tolerance before switching up your routine entirely.
Social media can make any chemical sound scary. A few influencers love to single out ingredients with long names. They jump straight to “toxic” without covering scientific research. Sodium isooctanoate has a short toxicity history, and everyday exposure levels in skincare don’t cross into dangerous territory.
There is always room for more testing. Kids, people with severe allergies, and those dealing with certain skin conditions need targeted research. Regulatory agencies tend to track these issues closely, so keeping an eye on updates from groups like the FDA or the European Commission adds an extra layer of reassurance.
Instead of memorizing a dictionary of ingredient names, I’ve found it smarter to know my skin’s triggers. Talking with a board-certified dermatologist beats trusting random claims from the internet. If you notice irritation, stop using the product and consult an expert. For most people, sodium isooctanoate does its job quietly and safely behind the scenes. Gentle surfactants keep cleansing accessible to everyone, including folks with drier or more sensitive skin, as long as formulas don’t stack up on additional harsh additives or fragrances. That’s the peace of mind that helps you walk away from the mirror feeling good about what’s in your routine.
Plenty of people checking the ingredients on their skincare or cleaning supplies come across sodium isooctanoate. It’s a compound made by neutralizing isooctanoic acid with sodium hydroxide. You’ll find it in cleansers, shampoos, and all sorts of chemical mixtures that need a surfactant to help oils and water mix, dissolve grime, or form a stable emulsion. Some shoppers, including my own friends and family, get nervous about complicated names. The big question they ask is: Is it natural or synthetic?
Isooctanoic acid, the main building block, doesn’t just drizzle out of tree sap or well water. It is found in very tiny amounts in some fermented foods or essential oils, but for almost every product you see, companies manufacture it through chemical processes. That doesn’t just mean tossing ingredients together in a vat; petrochemical feedstocks (like isooctene) get put through a series of reactions. Once technicians have this acid, they react it with sodium hydroxide (common lye)—and, frankly, lye doesn’t sprout on a bush either.
Every step of this process is guided by skilled chemists. Laboratories track purity and safety with sensitive equipment most people have never seen outside a classroom. There’s science and precision at each stage. Each batch gets checked because even tiny impurities can make a cosmetic or food product less reliable.
Big food and personal care companies love the word “natural.” There’s no law in the United States forcing a company to use a consistent definition. To a regular shopper, “natural” often means something that exists in the world, basically unchanged. But industry and science view things differently. If something comes from a plant or animal, that sounds natural to many, but start transforming it with heat, pressure, or chemical reactions, and it quickly looks synthetic to the public.
Sodium isooctanoate’s building blocks can be traced back to sources found in nature, but modern production relies almost entirely on synthetic routes using industrial chemistry. Its structure and behavior wind up the same as a theoretically “natural” sample, but the path there looks very different. So while companies sometimes claim it “occurs in nature,” the bottles on store shelves rely on synthetic versions.
Most folks just want to know if a product is safe. The real test is not whether an ingredient is natural. Plenty of natural poisons exist, and many synthetics are rigorously tested to keep people healthy. The U.S. Food and Drug Administration, Health Canada, the EU’s Scientific Committee on Consumer Safety—all these agencies set guidelines for testing, concentration, and usage in consumer products.
Sodium isooctanoate is considered safe for use in cosmetics and cleaners at typical concentrations. Research shows it poses little risk of irritation for most people. Dermatologists pay more attention to how a product performs—and whether it causes allergic reactions—than the origin of its carbon atoms. As someone who deals with sensitive skin, I care more about the test results and reliable manufacturing than a “natural” label.
If companies want to earn trust, honest and clear labeling matters more than buzzwords like “natural” or “green.” People deserve transparency. Regulations could follow the same logic: focus on safety, accurate sourcing, and full disclosure. Brands who educate shoppers about where ingredients come from—while providing solid science to back safety claims—will close the gap between fear and understanding. For sodium isooctanoate, synthetic might sound strange, but facts matter more than the label ever could.
Sodium isooctanoate pops up in lots of products, from laundry detergents and skincare to industrial cleaners. It acts as a surfactant—helping things mix—and does some heavy lifting for manufacturers looking for stable formulas. The ingredient comes from isooctanoic acid, which gets turned into a salt form that's more water-soluble and easier to use in everyday products.
People with sensitive or reactive skin know the value of checking ingredient lists. Contact allergies set off redness, itching, or a rash—not an experience you'll want from “gentle” cleansers. News stories and online forums occasionally talk about folks reacting to ingredients in detergents, prompting plenty to wonder about additives like sodium isooctanoate.
Right now, scientific reports on sodium isooctanoate are limited. The American Contact Dermatitis Society and North American Contact Dermatitis Group haven’t cited major outbreaks or case clusters tied to it. Most allergic reactions in soaps and cosmetics tend to trace back to added fragrances, preservatives like methylisothiazolinone, or dyes. Sodium isooctanoate itself shows a comparatively low risk for sensitization. Some lab studies and product safety reviews have run skin patch tests with this surfactant and didn’t see much evidence of allergic responses.
Allergists know that even low-allergy ingredients can't be ruled out completely. The skin’s natural barrier varies from person to person. Individuals with eczema (atopic dermatitis) or pre-existing allergies might notice irritation faster than others. Kids and older adults also show more sensitivity to some detergents. Everyone reacts a bit differently based on exposure history and the mix of other chemicals in a formula.
Helping family members with persistent hand rashes from dish soap, I’ve seen that cutting out fragrances and sticking with the simplest formulas can make a big difference. For instance, we eliminated products with plenty of extra “cleaning boosters,” switched to versions without added color or scent, and irritation cleared up in a few weeks. We stuck to gentle surfactants, though sodium isooctanoate itself wasn’t the main culprit. Skin reactions often have a snowball effect—one mild trigger primes the skin for bigger problems later on. A single new soap can tip the balance in sensitive folks.
Avoiding contact allergies means not just scanning for one ingredient, but considering the full recipe and your skin’s history with certain products. Those with known chemical sensitivities can benefit from patch-testing a small dab of a new soap or lotion before slathering it on. Reading up on published safety reviews for uncommon ingredients gives a sense of how common complaints are among a broader group, not just isolated anecdotes on the web.
For those with recurring eczema or diagnosed allergies, dermatologists typically recommend fragrance-free, dye-free products and keeping a symptom diary. Product makers could help by being even more transparent—listing every raw material, not just “main” ingredients—in language that’s simple for non-chemists. No one wants to lose a week of comfort from something as small as a laundry additive.
Scientists and consumer advocates keep pushing for thorough research and stronger regulations around all types of product additives. It’s not just about headline-grabbing allergens; subtle irritants add up in surprising ways. While sodium isooctanoate doesn’t seem to pose a widespread allergy problem right now, ongoing surveillance and informed choices help everyone stay safer—at home and at work.
Anyone noticing skin trouble from a household or personal care product should see a skin specialist for thorough patch testing. Staying proactive can help uncover the true triggers and steer families toward products that keep irritation to a minimum.
Sodium isooctanoate keeps showing up in ingredient lists for cleansers, micellar waters, and even wipes. Chemically, it acts as a surfactant that grabs on to oil and dirt, helping them to rinse away. It’s clear why formulators reach for it: this salt of a short-chain fatty acid lathers quickly, works in both hard and soft water, and doesn’t stick to the skin afterward.
The Mayo Clinic and the American Academy of Dermatology highlight the basics for sensitive skin: Steer clear of fragrances, harsh surfactants, and drying alcohols. Sodium isooctanoate gets classified as a “mild” surfactant. Researchers haven’t flagged it as a common allergen or irritant, which speaks in its favor compared to more notorious ingredients like sodium lauryl sulfate or benzalkonium chloride.
I’ve spent years navigating redness, stinging, and tightness after using the wrong products. Many in my shoes try to avoid pretty much anything that foams too vigorously or strips the barrier. Testing products with sodium isooctanoate felt different — there’s less of the immediate burning my skin often gives off if it senses trouble. Some of my friends with eczema or rosacea also report no problems, as long as the rest of the formula leaves out irritants or strong fragrances. The risk tends to climb if the product combines it with other aggressive cleansers or essential oils.
Studies and patch tests point in a positive direction. The Journal of the American College of Toxicology notes that sodium isooctanoate turns up low scores for skin irritancy, even in rinse-off formats. No dramatic outbreaks or rashes came up across multiple trials. That said, these weren’t huge studies with every skin type, so there’s still a chance that some people with extra-reactive skin might experience a setback. Skin barrier function depends on so many factors, from genetics to climate, making blanket statements difficult.
Marketing can give sodium isooctanoate a sort of “safe for all” halo, but it isn’t magic. If a company loads up a product with preservatives, synthetic fragrance, or other cheap surfactants, the main surfactant can’t save the day. Overuse also causes problems — washing three or four times a day, even with milder ingredients, wears down that fragile skin barrier. I learned that lesson the hard way after one winter filled with red, flaky skin from over-cleansing.
Patch testing gives answers that ingredient decks and online forums can’t. Dabbing a small amount of the new cleanser on the inside of an elbow for a few days picks up on hidden allergies you might not see coming. Reading reviews from others who struggle with sensitive skin also helps, since they tend to be brutally honest about what stings or soothes.
Using a short, ingredient-light product list keeps things simple. I’ve noticed my skin calms down if I cut back to a gentle cleanser, a bland moisturizer, and sunscreen. Products with sodium isooctanoate, few fillers, and no added perfume sit OK with me, but that’s only one data point. A good dermatologist will always be a better guide than any bottle in the discount section.
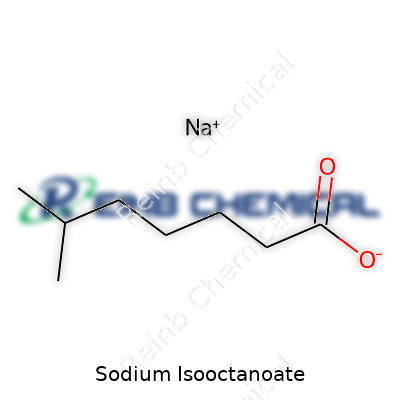
| Names | |
| Preferred IUPAC name | Sodium 6-methylheptanoate |
| Other names |
Sodium 2-ethylhexanoate Sodium isooctanoic acid Sodium octanate Sodium octanoate Sodium 2-ethylcaproate |
| Pronunciation | /ˈsəʊdiəm aɪsəʊˈɒktəˌneɪt/ |
| Identifiers | |
| CAS Number | [6106-41-8] |
| 3D model (JSmol) | `CC(C)CC(C)(C)C(=O)[O-].[Na+]` |
| Beilstein Reference | 1900966 |
| ChEBI | CHEBI:85274 |
| ChEMBL | CHEMBL4451202 |
| ChemSpider | 143609 |
| DrugBank | DB11093 |
| ECHA InfoCard | ECHA InfoCard: 100_009_899 |
| EC Number | EC 265-111-2 |
| Gmelin Reference | 110120 |
| KEGG | C14319 |
| MeSH | D013485 |
| PubChem CID | 102181 |
| RTECS number | WH0700000 |
| UNII | T40OKT44G0 |
| UN number | UN3276 |
| CompTox Dashboard (EPA) | DTXSID3039242 |
| Properties | |
| Chemical formula | C8H15NaO2 |
| Molar mass | 166.23 g/mol |
| Appearance | White powder |
| Odor | Odorless |
| Density | 0.96 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.75 |
| Vapor pressure | Negligible |
| Acidity (pKa) | pKa ≈ 4.8 |
| Basicity (pKb) | 10.50 |
| Refractive index (nD) | 1.4200 |
| Viscosity | Liquid |
| Dipole moment | 3.8818 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 226.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -706.46 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4758.4 kJ/mol |
| Pharmacology | |
| ATC code | S0005 |
| Hazards | |
| Main hazards | Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD50 Oral Rat 3600 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral >2000 mg/kg |
| PEL (Permissible) | PEL (Permissible) for Sodium Isooctanoate: Not specifically established by OSHA |
| REL (Recommended) | 2500 mg/L |
| Related compounds | |
| Related compounds |
Sodium octanoate Isooctanoic acid Octanoic acid Potassium isooctanoate Sodium 2-ethylhexanoate |